Radiologic Physics Nuclear Medicine PET Imaging and Quantification













- Slides: 47

Radiologic Physics: Nuclear Medicine PET Imaging and Quantification Suleman Surti surti@mail. med. upenn. edu (215) 662 -7214

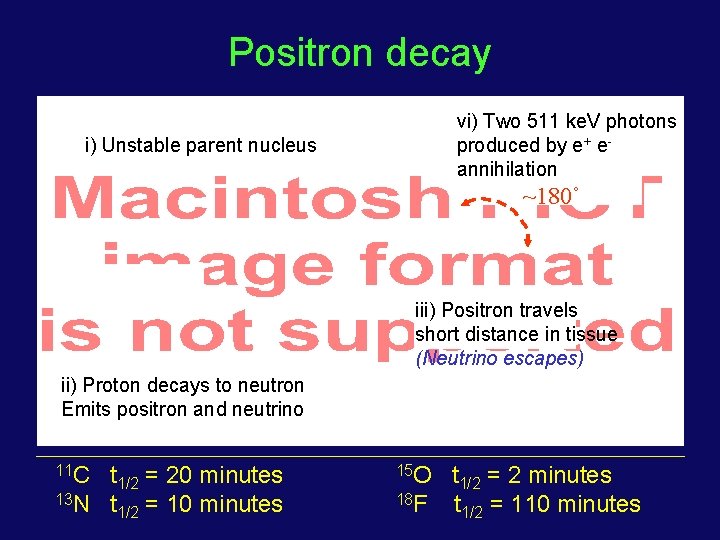
Positron decay vi) Two 511 ke. V photons produced by e+ eannihilation i) Unstable parent nucleus ~180˚ iii) Positron travels short distance in tissue (Neutrino escapes) ii) Proton decays to neutron Emits positron and neutrino 11 C t 1/2 = 20 minutes 13 N t 1/2 = 10 minutes 15 O t 1/2 = 2 minutes 18 F t 1/2 = 110 minutes

Positron Emission Tomography A primary goal and usefulness of a tomographic imaging modality such as PET is to achieve images where the intensity of each voxel in the image is proportional to the activity concentration present in the corresponding location in the patient

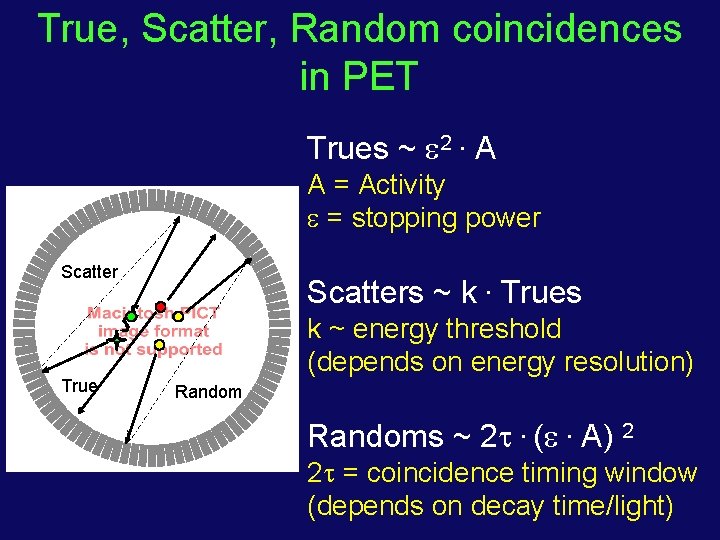
True, Scatter, Random coincidences in PET Trues ~ 2. A A = Activity = stopping power Scatter True Scatters ~ k. Trues k ~ energy threshold (depends on energy resolution) Randoms ~ 2 . ( . A) 2 2 = coincidence timing window (depends on decay time/light)

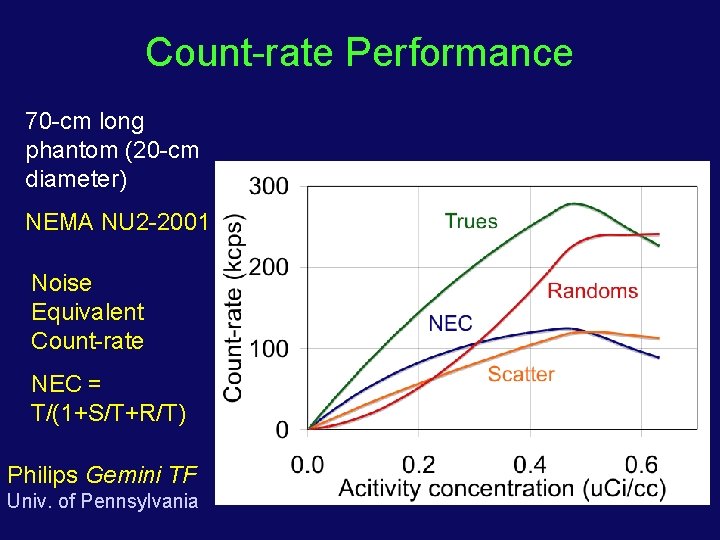
Count-rate Performance 70 -cm long phantom (20 -cm diameter) NEMA NU 2 -2001 Noise Equivalent Count-rate NEC = T/(1+S/T+R/T) Philips Gemini TF Univ. of Pennsylvania

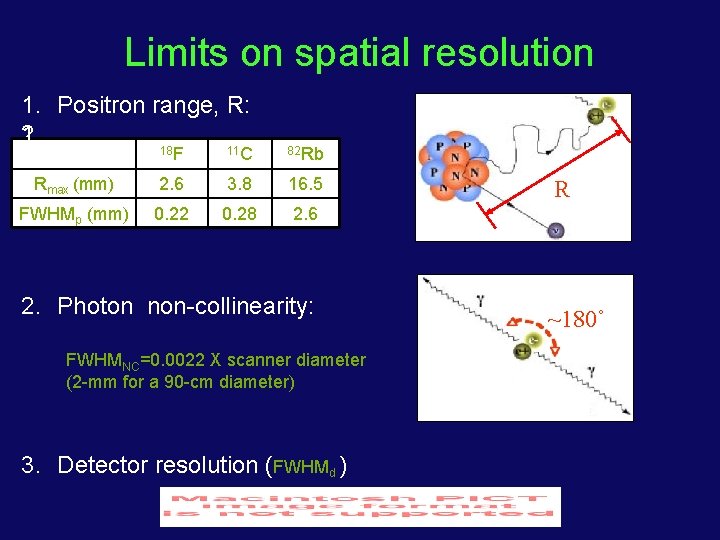
Limits on spatial resolution 1. Positron range, R: 2. 1. 18 F 11 C 82 Rb Rmax (mm) 2. 6 3. 8 16. 5 FWHMp (mm) 0. 22 0. 28 2. 6 2. Photon non-collinearity: FWHMNC=0. 0022 X scanner diameter (2 -mm for a 90 -cm diameter) 3. Detector resolution (FWHMd ) R ~180˚

PET Instrumentation Design • Scintillators stopping power, speed, light output • Detector configuration scintillator - photo-sensor coupling • Scanner geometry field-of-view (axial) 2 -dimensional vs. 3 -dimensional Time-of-flight PET • Data processing / image reconstruction scatter, randoms and attenuation correction iterative reconstruction algorithms

Comparison of Scintillators

Scintillation Detector Photo-Multiplier Tube (PMT) Scintillator

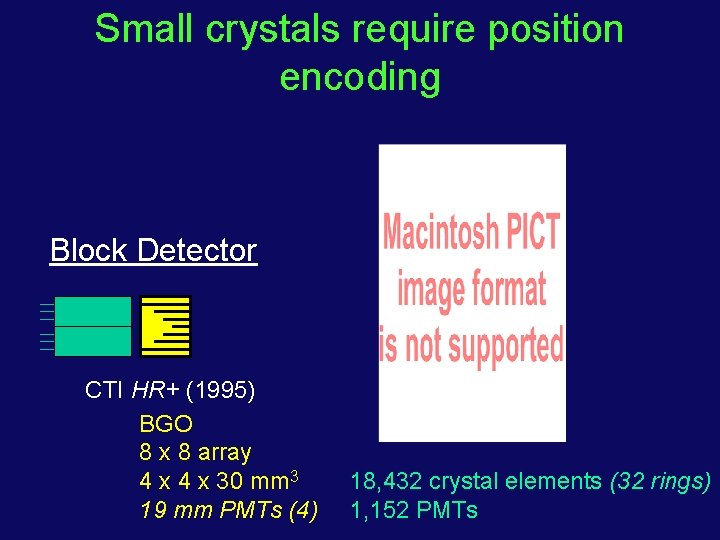
Small crystals require position encoding Block Detector CTI HR+ (1995) BGO 8 x 8 array 4 x 30 mm 3 19 mm PMTs (4) 18, 432 crystal elements (32 rings) 1, 152 PMTs

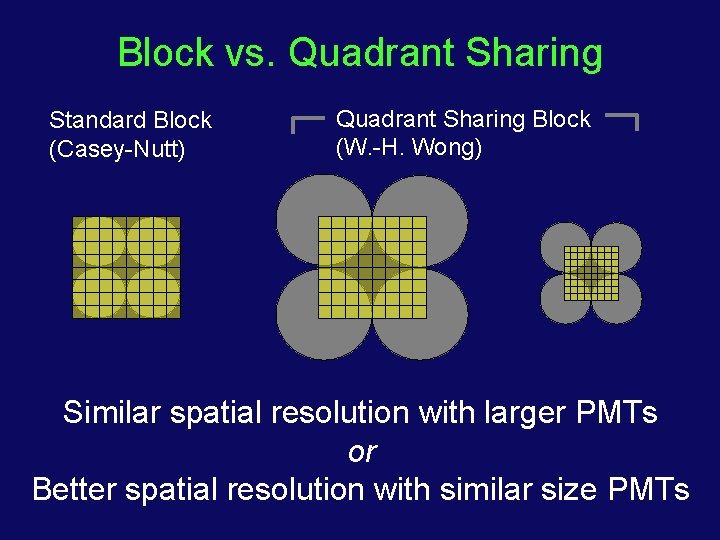
Block vs. Quadrant Sharing Standard Block (Casey-Nutt) Quadrant Sharing Block (W. -H. Wong) Similar spatial resolution with larger PMTs or Better spatial resolution with similar size PMTs

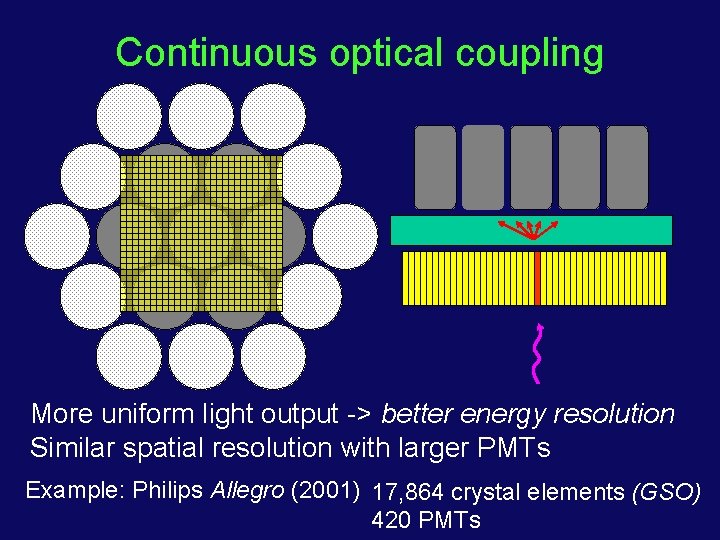
Continuous optical coupling More uniform light output -> better energy resolution Similar spatial resolution with larger PMTs Example: Philips Allegro (2001) 17, 864 crystal elements (GSO) 420 PMTs

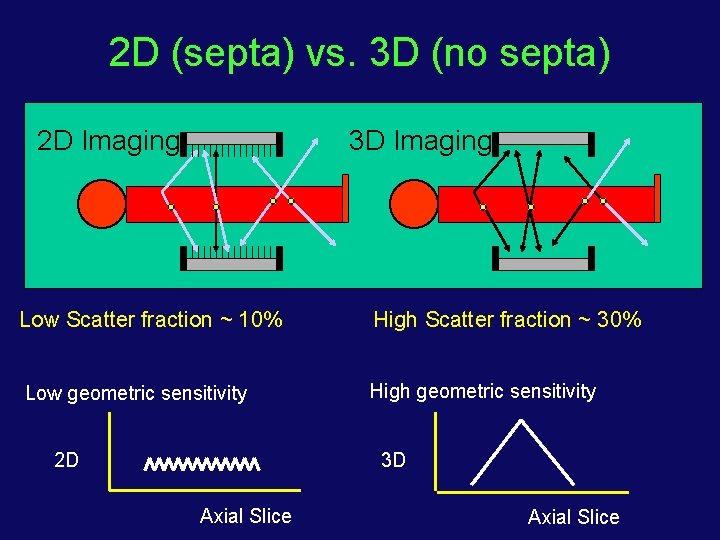
2 D (septa) vs. 3 D (no septa) 2 D Imaging 3 D Imaging Low Scatter fraction ~ 10% High Scatter fraction ~ 30% Low geometric sensitivity High geometric sensitivity 2 D 3 D Axial Slice

Energy threshold reduces scatter & random coincidences- particularly in 3 D Scatter/True=k Scatter/True>k True

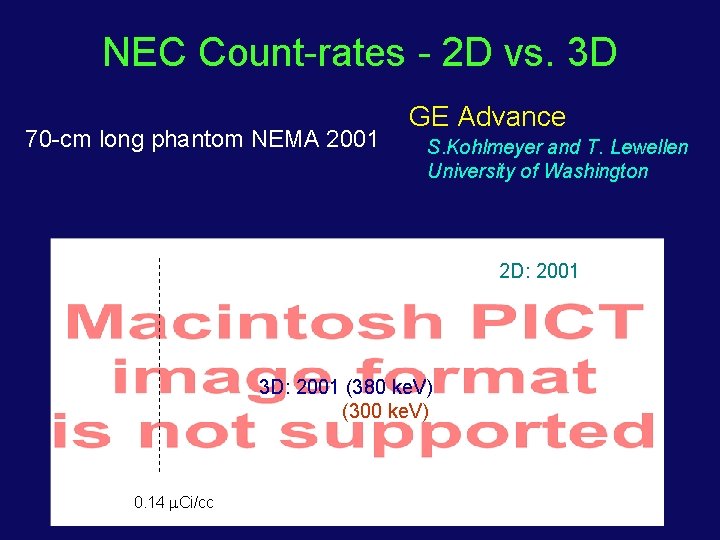
NEC Count-rates - 2 D vs. 3 D 70 -cm long phantom NEMA 2001 GE Advance S. Kohlmeyer and T. Lewellen University of Washington 2 D: 2001 3 D: 2001 (380 ke. V) (300 ke. V) 0. 14 Ci/cc

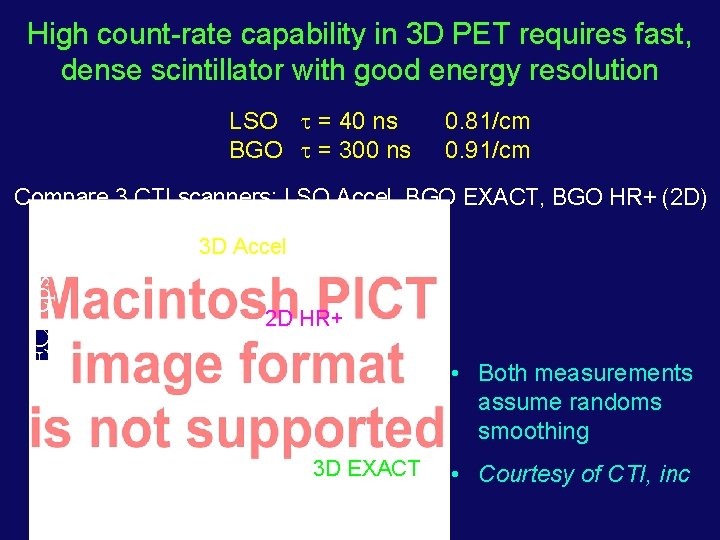
High count-rate capability in 3 D PET requires fast, dense scintillator with good energy resolution LSO = 40 ns BGO = 300 ns 0. 81/cm 0. 91/cm Compare 3 CTI scanners: LSO Accel, BGO EXACT, BGO HR+ (2 D) NEC (cps) 3 D Accel 2 D HR+ • Both measurements assume randoms smoothing 3 D EXACT • Courtesy of CTI, inc

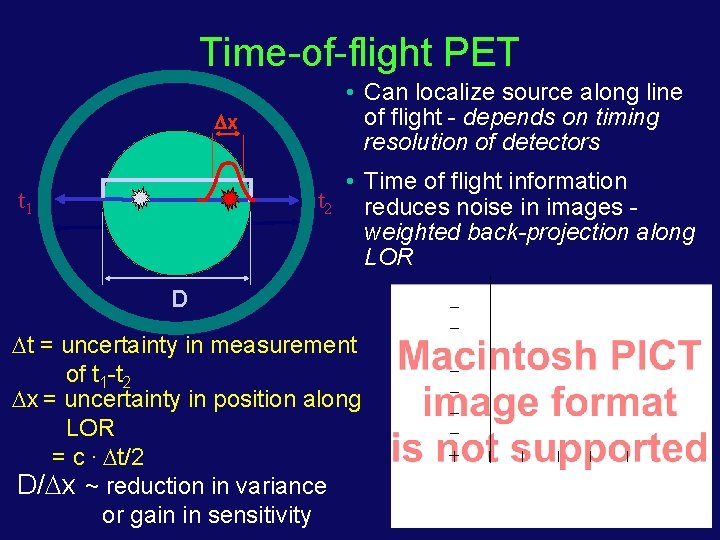
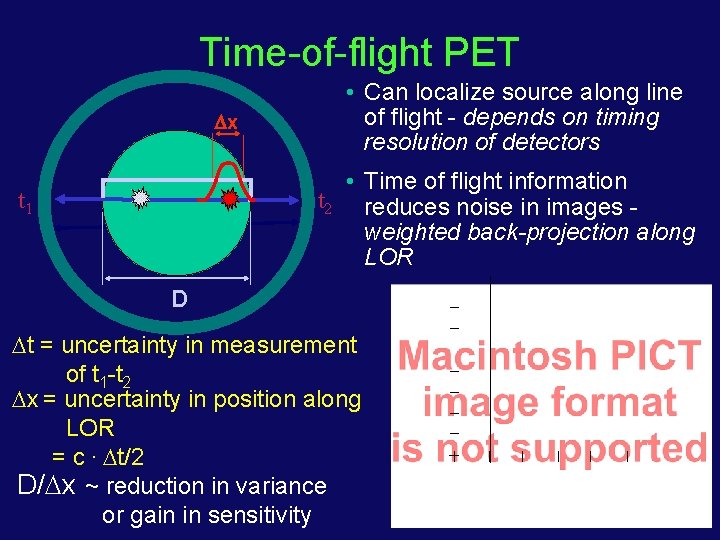
Time-of-flight PET x • Can localize source along line of flight - depends on timing resolution of detectors • Time of flight information t 2 reduces noise in images weighted back-projection along LOR t 1 D t = uncertainty in measurement of t 1 -t 2 x = uncertainty in position along LOR = c. t/2 D/ x ~ reduction in variance or gain in sensitivity

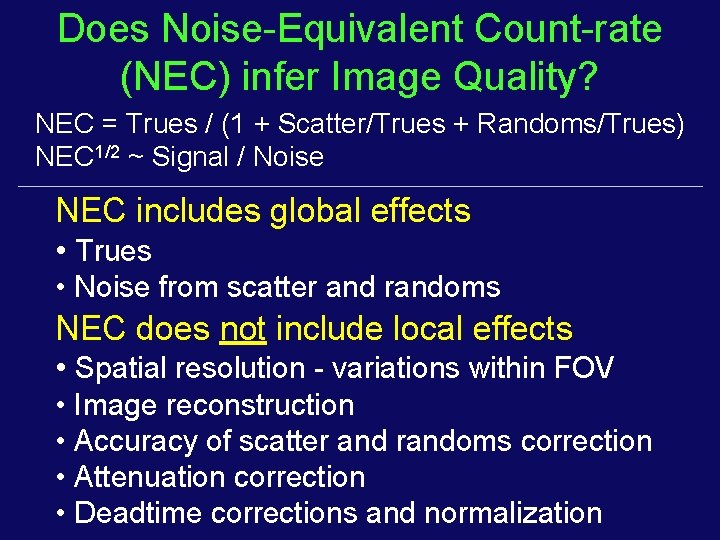
Does Noise-Equivalent Count-rate (NEC) infer Image Quality? NEC = Trues / (1 + Scatter/Trues + Randoms/Trues) NEC 1/2 ~ Signal / Noise NEC includes global effects • Trues • Noise from scatter and randoms NEC does not include local effects • Spatial resolution - variations within FOV • Image reconstruction • Accuracy of scatter and randoms correction • Attenuation correction • Deadtime corrections and normalization

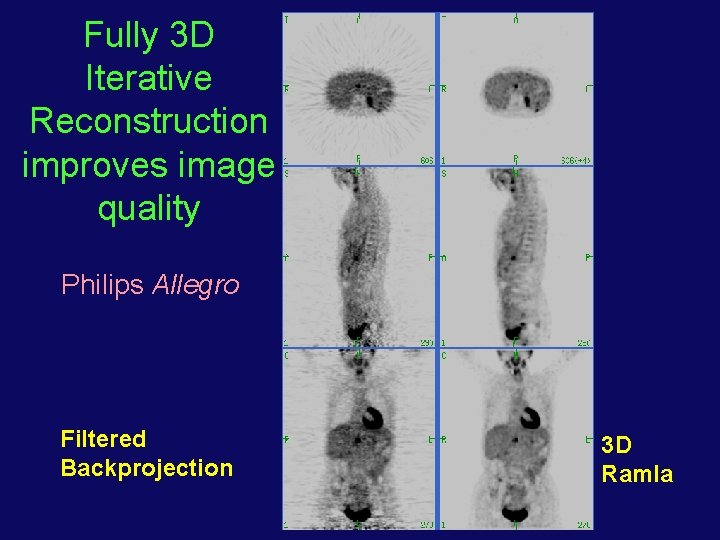
Fully 3 D Iterative Reconstruction improves image quality Philips Allegro Filtered Backprojection 3 D Ramla

Positron Emission Tomography What is needed to achieve quantitative PET images? 1. 2. 3. 4. 5. Deadtime correction Data Normalization Scatter correction Randoms correction Attenuation correction

Deadtime correction • Deadtime — High count-rate effect present in radiation detectors • Two manifestations: • Pulse pileup — Events are collected but measurements such as energy and spatial position are affected (reduced image quality) • Loss of counts — Due to electronics deadtime and determined mainly by scintillator decay time • Loss of counts corrected by measuring collected counts vs activity in a uniform cylinder

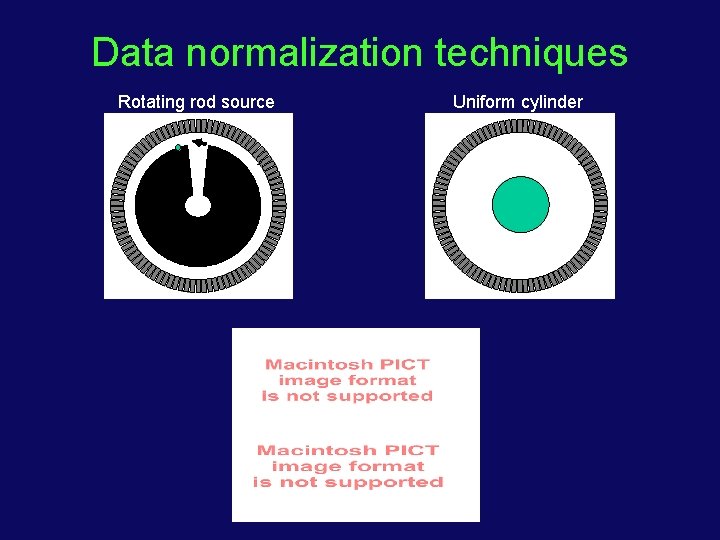
Data normalization • Normalization — non-uniformities in event detection over the full scanner • Two sources: • Variation in amount of scintillation light collection due to crystal nonuniformities and detector design (detector effect) • Difference in detection sensitivity due to angle of incidence >d d

Data normalization techniques Rotating rod source Uniform cylinder

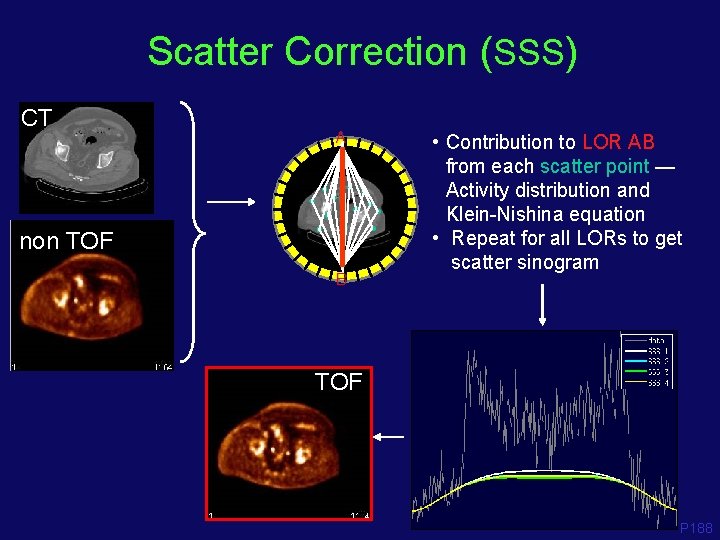
Scatter Correction (SSS) CT A non TOF B • Contribution to LOR AB from each scatter point — Activity distribution and Klein-Nishina equation • Repeat for all LORs to get scatter sinogram TOF P 188

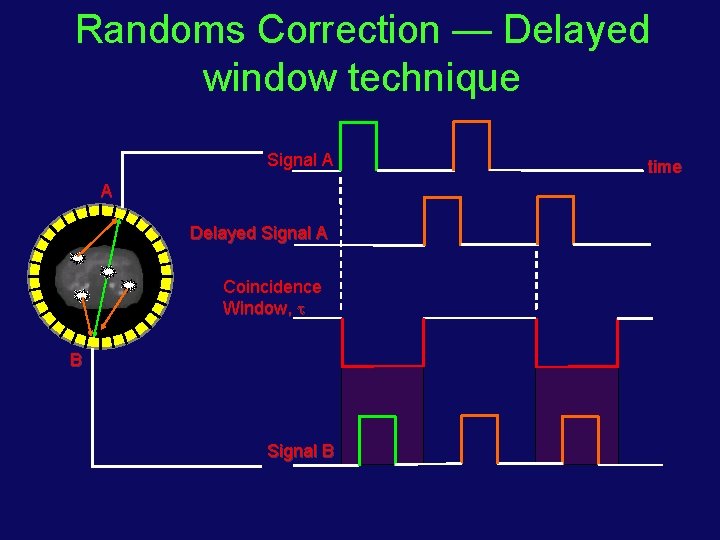
Randoms Correction — Delayed window technique Signal A A Delayed Signal A Coincidence Window, B Signal B time

Why do we need attenuation correction? • More accurate activity distribution uniform liver, ‘cold lungs’ • Improved lesion detectability deep lesions • Reduce image artifacts and streaking reconstruct using consistent data • Improved image quality with iterative reconstruction include attenuation into model But…attenuation correction must be FAST - compared to emission scan ACCURATE - e. g. near lung boundary LOW NOISE - minimize noise propagation

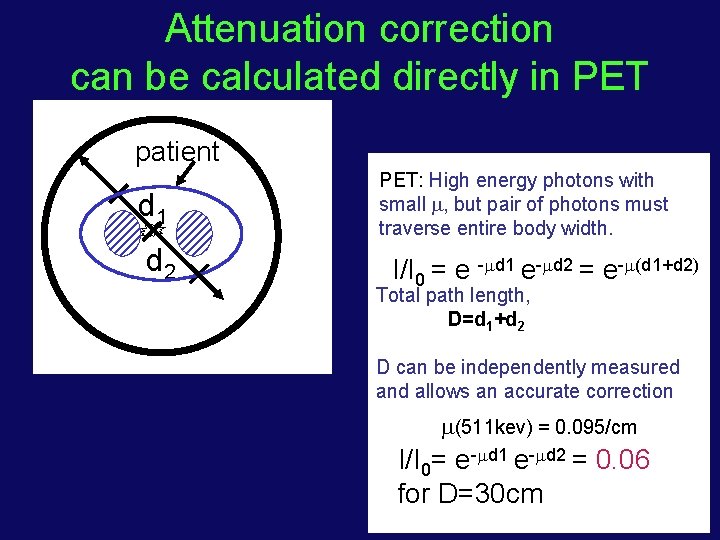
Attenuation correction can be calculated directly in PET patient d 1 d 2 PET: High energy photons with small , but pair of photons must traverse entire body width. I/I 0 = e - d 1 e- d 2 = e- (d 1+d 2) Total path length, D=d 1+d 2 D can be independently measured and allows an accurate correction (511 kev) = 0. 095/cm I/I 0= e- d 1 e- d 2 = 0. 06 for D=30 cm

Transmission sources for attenuation measurements 1. PET transmission source (68 Ge/68 Ga) - source of coincident annihilation photons (mono energetic @ 511 ke. V), 265 day half life 2. Single photon source (137 Cs) - source of single -rays (mono energetic @ 662 ke. V), 20 yr half-life 3. X-ray CT scan - source of X-rays with a distribution of energies from ~30 to 120 ke. V. We can assume an ‘effective’ energy of ~ 75 ke. V spectra positron source -ray source X-ray source Intensity I 0(E) 0 30 120 E (ke. V) 511 662 (Recall that the PET emission data is attenuated at 511 ke. V)

Transmission Scan d 1 d 2 d 1 + d 2 = D Emission I / I 0 = e- d 1. e- d 2 = e- D Transmission I / I 0 = e- D 137 Cs point source 662 ke. V, t 1/2 = 30 yr

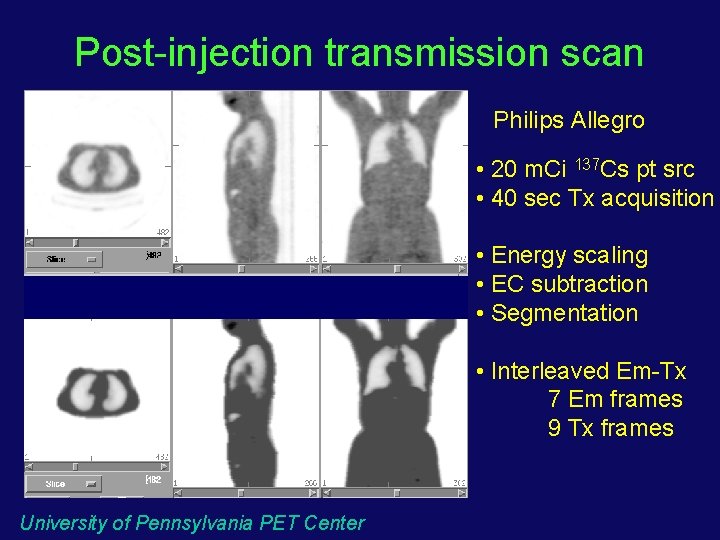
Post-injection transmission scan Philips Allegro • 20 m. Ci 137 Cs pt src • 40 sec Tx acquisition • Energy scaling • EC subtraction • Segmentation • Interleaved Em-Tx 7 Em frames 9 Tx frames University of Pennsylvania PET Center

CT-based attenuation correction: threshold method STEP 1: Separate bone and soft tissue using threshold of 300 H. U. STEP 2: Scale to PET energy 511 ke. V. Scale factors (511: ~70 ke. V): bone 0. 41, soft tissue: 0. 50 STEP 3: Forward project to obtain attenuation correction factors. Kinahan PE, Townsend DW, Beyer T, et al. Med Phys. 1998; 25(10): 2046 -2053.

Potential problems for CT-based attenuation correction • Difference in CT and PET respiratory patterns Can lead to artifacts near the dome of the liver • Use of contrast agent Can cause incorrect values in PET image • Truncation of CT image due to keeping arms down in the field of view to match the PET scan Can cause artifacts in corresponding regions in PET image • Bias in the CT image due to beam-hardening and scatter from the arms in the field of view

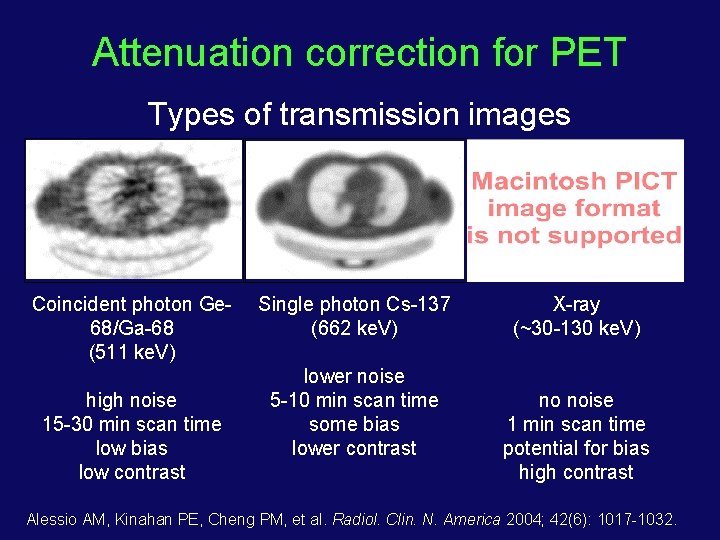
Attenuation correction for PET Types of transmission images Coincident photon Ge 68/Ga-68 (511 ke. V) high noise 15 -30 min scan time low bias low contrast Single photon Cs-137 (662 ke. V) lower noise 5 -10 min scan time some bias lower contrast X-ray (~30 -130 ke. V) no noise 1 min scan time potential for bias high contrast Alessio AM, Kinahan PE, Cheng PM, et al. Radiol. Clin. N. America 2004; 42(6): 1017 -1032.

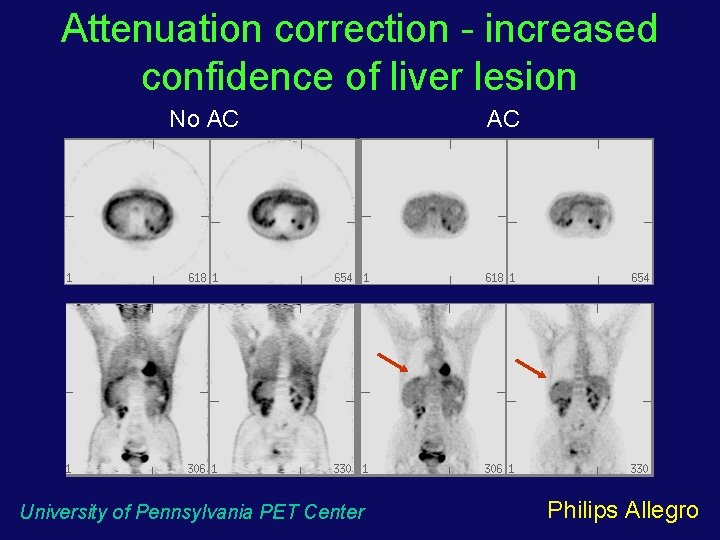
Attenuation correction - increased confidence of liver lesion No AC University of Pennsylvania PET Center AC Philips Allegro

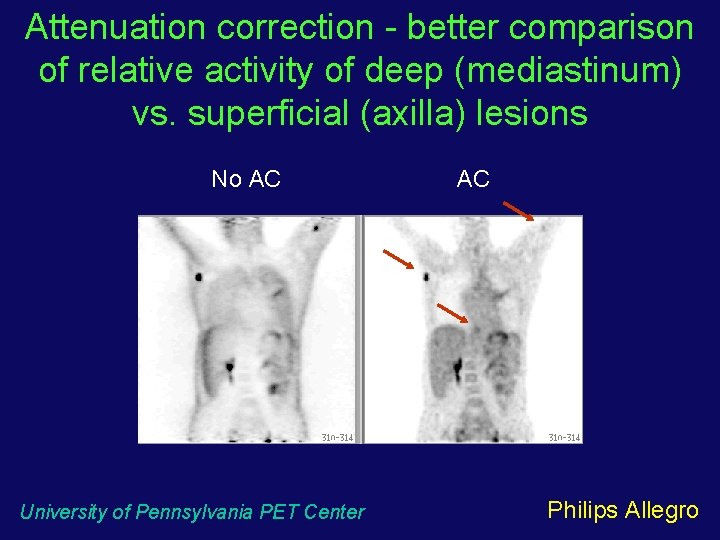
Attenuation correction - better comparison of relative activity of deep (mediastinum) vs. superficial (axilla) lesions No AC University of Pennsylvania PET Center AC Philips Allegro

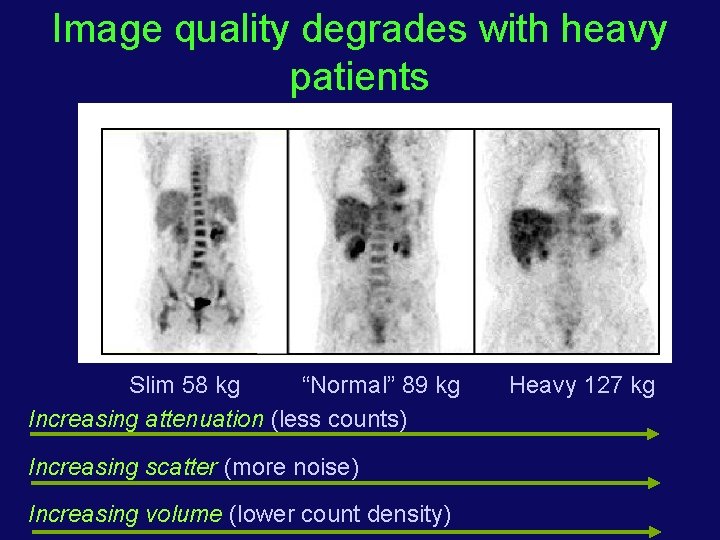
Image quality degrades with heavy patients Slim 58 kg “Normal” 89 kg Increasing attenuation (less counts) Increasing scatter (more noise) Increasing volume (lower count density) Heavy 127 kg

How can we improve image quality? 2 D - counts limited by septa and maximum allowed dose 3 D - counts limited by dead-time and randoms Scintillator High stopping power - higher coincidence fraction Fast decay - lower dead-time and randoms Energy resolution - lower scatter and randoms Geometry Sensitivity ~ (Axial FOV)2 (increased scintillator and PMT cost) Time-of-flight Requires very fast scintillator with excellent timing resolution

Time-of-flight PET x • Can localize source along line of flight - depends on timing resolution of detectors • Time of flight information t 2 reduces noise in images weighted back-projection along LOR t 1 D t = uncertainty in measurement of t 1 -t 2 x = uncertainty in position along LOR = c. t/2 D/ x ~ reduction in variance or gain in sensitivity

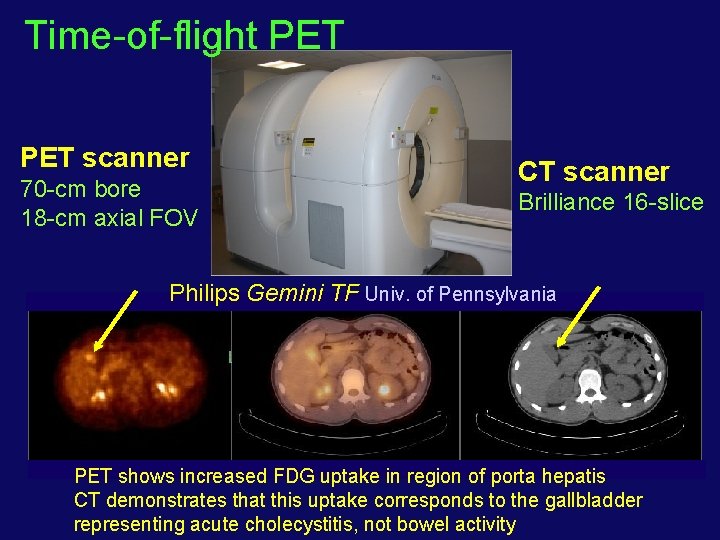
Time-of-flight PET scanner 70 -cm bore 18 -cm axial FOV CT scanner Brilliance 16 -slice Philips Gemini TF Univ. of Pennsylvania PET shows increased FDG uptake in region of porta hepatis CT demonstrates that this uptake corresponds to the gallbladder representing acute cholecystitis, not bowel activity

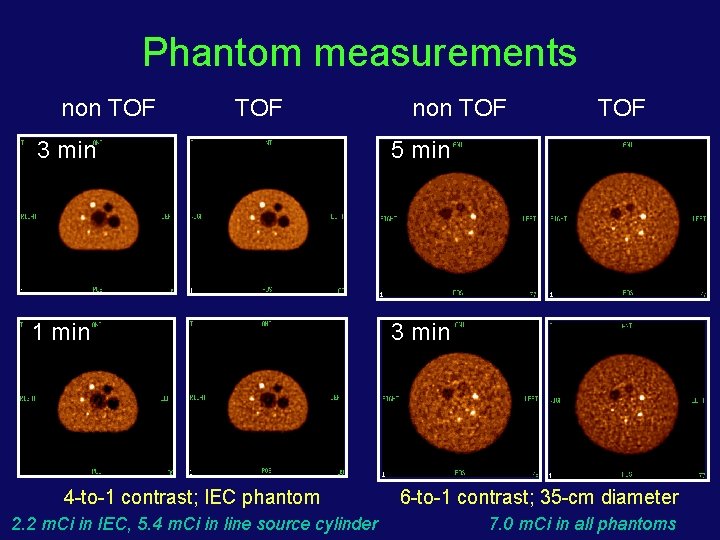
Phantom measurements non TOF 3 min 5 min 1 min 3 min 4 -to-1 contrast; IEC phantom 2. 2 m. Ci in IEC, 5. 4 m. Ci in line source cylinder TOF 6 -to-1 contrast; 35 -cm diameter 7. 0 m. Ci in all phantoms

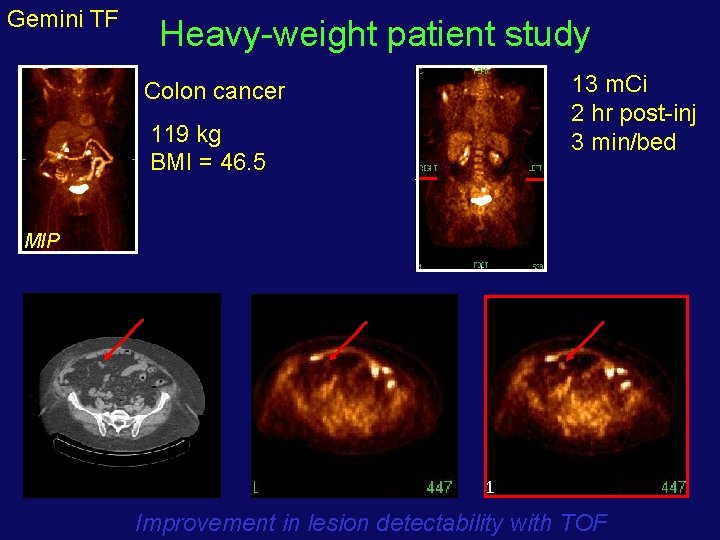
Gemini TF Heavy-weight patient study 13 m. Ci 2 hr post-inj 3 min/bed Colon cancer 119 kg BMI = 46. 5 MIP LDCT non-TOF Improvement in lesion detectability with TOF

Clinical 18 F-FDG imaging • Clinical 18 F-FDG imaging essentially involves two tasks: • Identifying regions with abnormal uptake (lesion detection) • Deriving a measure of glucose metabolism in these regions (lesion estimation task)

Factors affecting lesion detection and activity estimation • Accuracy of scanner normalization and corrections for deadtime, scatter, randoms, & attenuation • Remove biases with minimal noise propagation • Spatial resolution • Lesion size and partial volume effects • Lesion activity uptake relative to background • Scan time • Reduced noise • Patient habitus • Determines amount of Sc, R, and attenuation • Reconstruction • Determines amount of noise in image and for iterative algorithms plays off contrast recovery with noise

Summary • PET scanner design is still an evolving area of research with new scintillators and photo-detectors being developed • Current generation of clinical scanners achieve spatial resolution of 4 -5 mm • Fully-3 D imaging is imaging mode of choice • PET is still count limited • TOF PET can help improve the statistical quality of PET images • PET/CT as a multi-modality imaging device has increased the confidence in interpreting PET images • Future direction - PET/MRI scanners

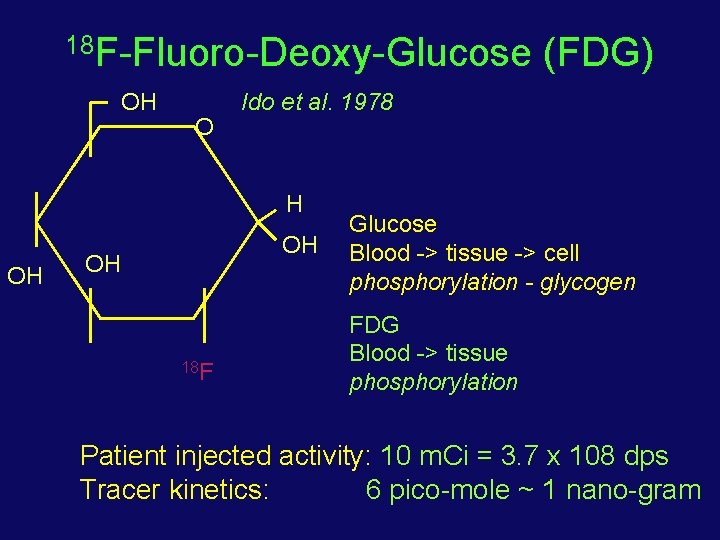
18 F-Fluoro-Deoxy-Glucose OH O Ido et al. 1978 H OH OH OH 18 F (FDG) Glucose Blood -> tissue -> cell phosphorylation - glycogen FDG Blood -> tissue phosphorylation Patient injected activity: 10 m. Ci = 3. 7 x 108 dps Tracer kinetics: 6 pico-mole ~ 1 nano-gram

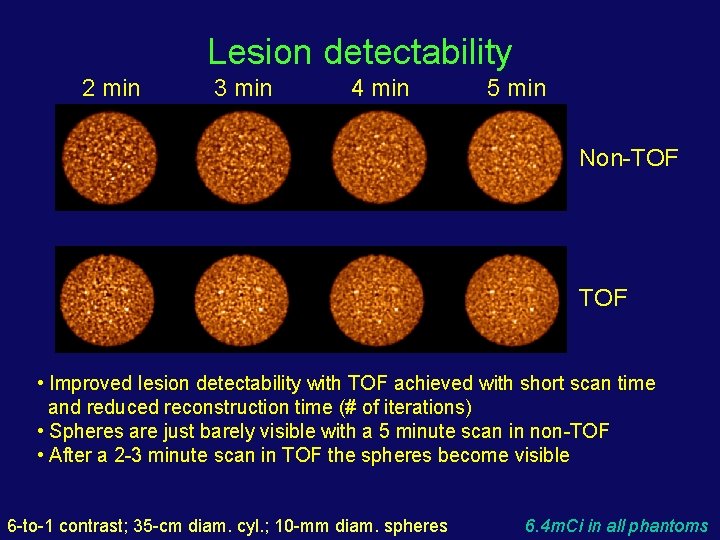
Lesion detectability 2 min 3 min 4 min 5 min Non-TOF • Improved lesion detectability with TOF achieved with short scan time and reduced reconstruction time (# of iterations) • Spheres are just barely visible with a 5 minute scan in non-TOF • After a 2 -3 minute scan in TOF the spheres become visible 6 -to-1 contrast; 35 -cm diam. cyl. ; 10 -mm diam. spheres 6. 4 m. Ci in all phantoms

Time-of-flight scanners need investigation of new data processing and image reconstruction methods • Scatter correction - can incorporate timing information - energy based methods - statistical weighting • Image reconstruction - list-mode ML-EM - optimize use of TOF - include data corrections in system model - spatial recovery • Data quantification - SUV estimation - convergence of lesion contrast improves with TOF • Image evaluation - lesion detectability measures - how does TOF improve SNR in image?