Radioactivity What do we mean by Radioactivity Radioactive

Radioactivity

What do we mean by Radioactivity? Radioactive decay is the process in which an unstable atomic nucleus loses energy by emitting radiation in the form of particles or electromagnetic waves. There are numerous types of radioactive decay. The general idea: An unstable nucleus releases energy to become more stable

Early Pioneers in Radioactivity Rutherford: Roentgen: Discoverer Alpha and Beta rays 1897 Discoverer of X-rays 1895 The Curies: Discoverers of Radium and Polonium 19001908 Becquerel: Discoverer of Radioactivity 1896
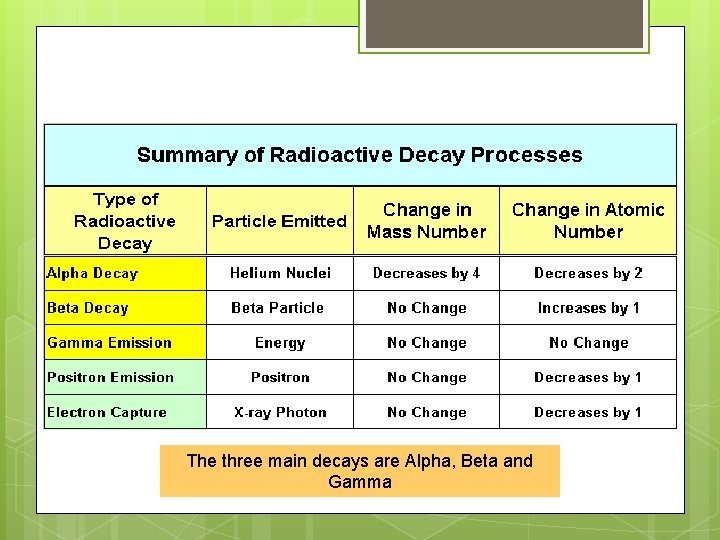
Kinds of Radioactivity The three main decays are Alpha, Beta and Gamma
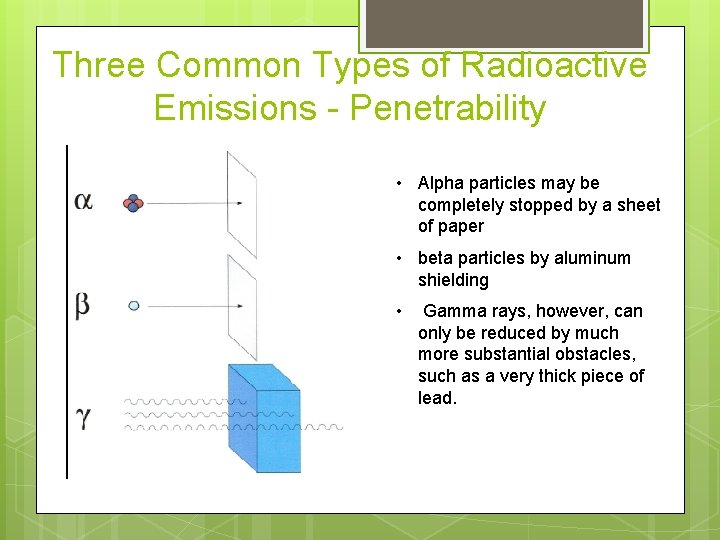
Three Common Types of Radioactive Emissions - Penetrability • Alpha particles may be completely stopped by a sheet of paper • beta particles by aluminum shielding • Gamma rays, however, can only be reduced by much more substantial obstacles, such as a very thick piece of lead.

Sources of Radioactivity Primordial - from before the creation of the Earth Cosmogenic - formed as a result of cosmic ray interactions Human produced - enhanced or formed due to human actions (minor amounts compared to natural)
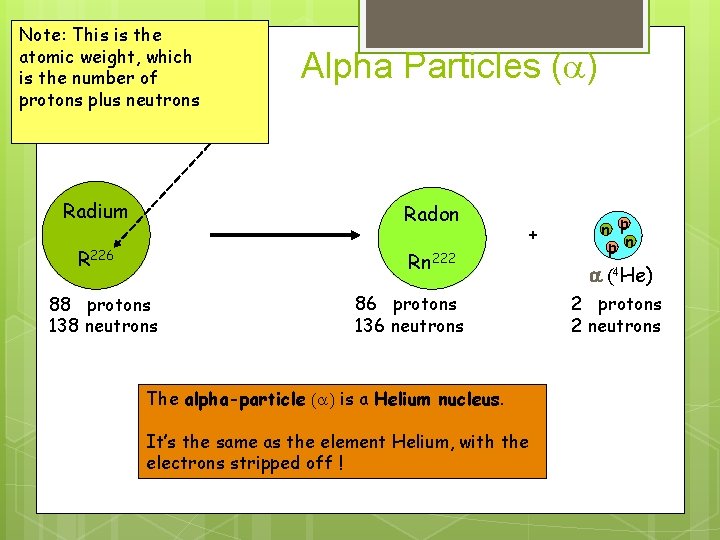
Note: This is the atomic weight, which is the number of protons plus neutrons Alpha Particles (a) Radium Radon R 226 Rn 222 88 protons 138 neutrons + 86 protons 136 neutrons The alpha-particle (a) is a Helium nucleus. It’s the same as the element Helium, with the electrons stripped off ! n p p n a (4 He) 2 protons 2 neutrons
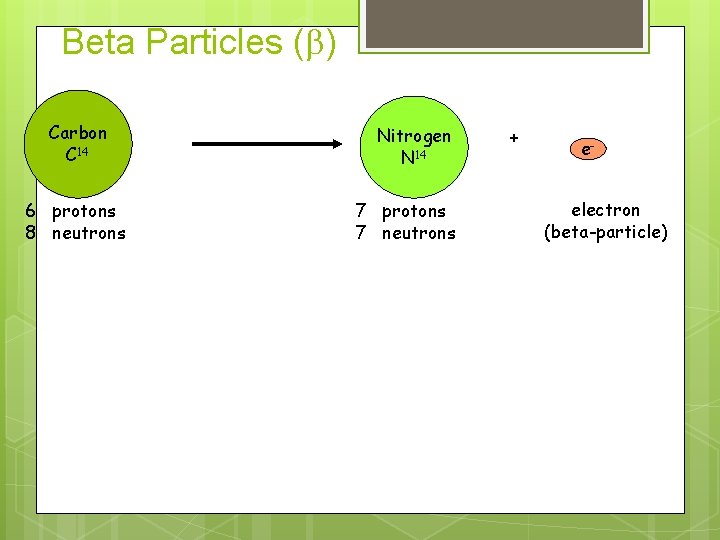
Beta Particles (b) Carbon C 14 6 protons 8 neutrons Nitrogen N 14 7 protons 7 neutrons + eelectron (beta-particle)
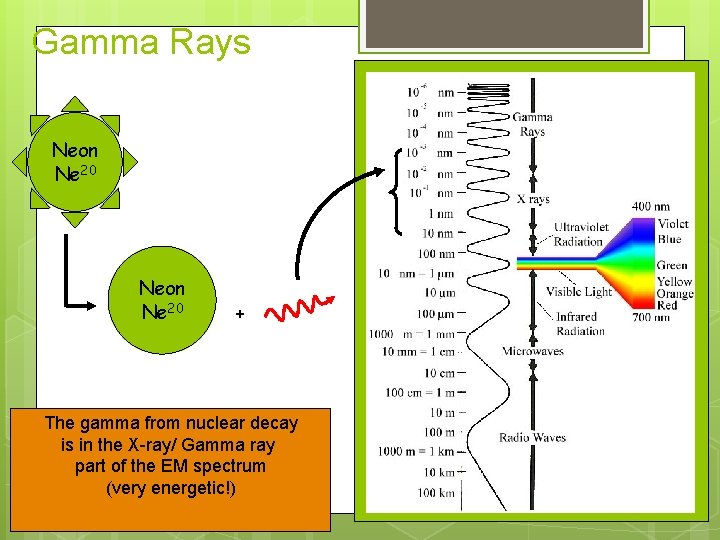
Gamma Rays Neon Ne 20 + The gamma from nuclear decay is in the X-ray/ Gamma ray part of the EM spectrum (very energetic!)

Bismuth – 214 decays to produce an isotope and an alpha particle. Write the reaction.
- Slides: 10