Radioactivity Radioactivity Radiation Radioactivity The process by which

Radioactivity
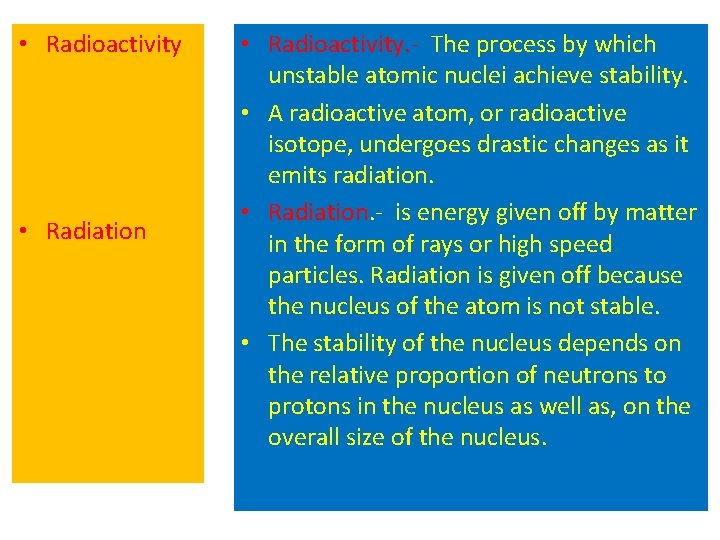
• Radioactivity • Radiation • Radioactivity. - The process by which unstable atomic nuclei achieve stability. • A radioactive atom, or radioactive isotope, undergoes drastic changes as it emits radiation. • Radiation. - is energy given off by matter in the form of rays or high speed particles. Radiation is given off because the nucleus of the atom is not stable. • The stability of the nucleus depends on the relative proportion of neutrons to protons in the nucleus as well as, on the overall size of the nucleus.
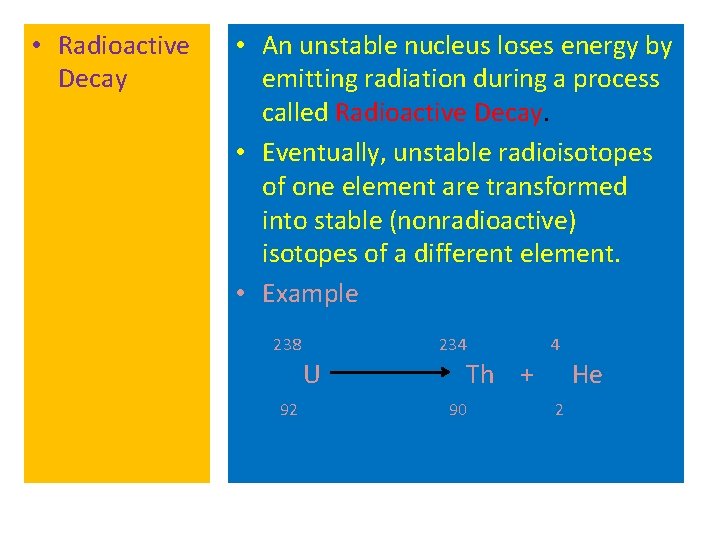
• Radioactive Decay • An unstable nucleus loses energy by emitting radiation during a process called Radioactive Decay. • Eventually, unstable radioisotopes of one element are transformed into stable (nonradioactive) isotopes of a different element. • Example 238 234 U 92 4 Th + 90 He 2

• Radioactive decay is spontaneous and does not require any input of energy. The rate of radioactive decay is unaffected by temperature, pressure or a catalyst. • Radioactive decay happens at a constant rate.

• Radioactivity • Radiation • Atoms are made up of protons and neutrons located in the nucleus of the atom. The nucleus has a positive charge. The atom outershell contains the negative charged electrons. • Nuclear forces keep the nucleus strong, balanced, and stable by getting rid of excess atomic energy (Radioactivity). • In that process, an unstable nuclei may emit a quantity of energy. This spontaneous emission of energy is what we call radiation.
- Slides: 5