Radioactivity Law of Decay Half Life and Statistics








- Slides: 19

Radioactivity Law of Decay, Half Life and Statistics Weak 3 Dr. Hanan Y. Abbas- dhmd 70@hotmail. com 1

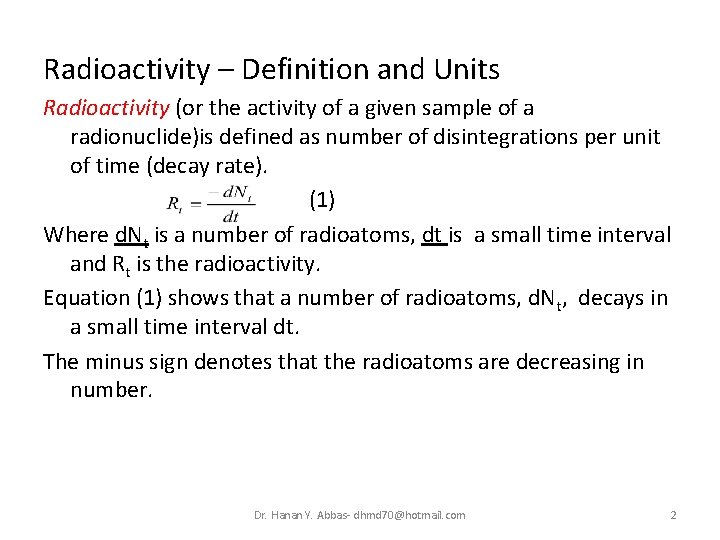
Radioactivity – Definition and Units Radioactivity (or the activity of a given sample of a radionuclide)is defined as number of disintegrations per unit of time (decay rate). (1) Where d. Nt is a number of radioatoms, dt is a small time interval and Rt is the radioactivity. Equation (1) shows that a number of radioatoms, d. Nt, decays in a small time interval dt. The minus sign denotes that the radioatoms are decreasing in number. Dr. Hanan Y. Abbas- dhmd 70@hotmail. com 2

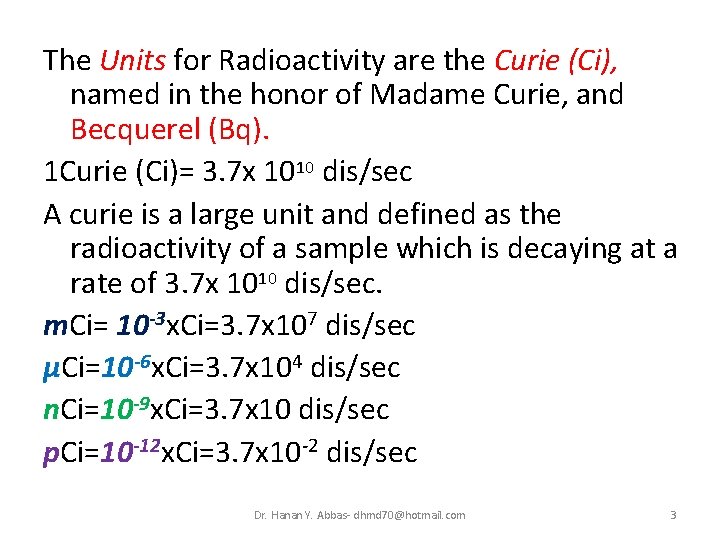
The Units for Radioactivity are the Curie (Ci), named in the honor of Madame Curie, and Becquerel (Bq). 1 Curie (Ci)= 3. 7 x 1010 dis/sec A curie is a large unit and defined as the radioactivity of a sample which is decaying at a rate of 3. 7 x 1010 dis/sec. m. Ci= 10 -3 x. Ci=3. 7 x 107 dis/sec μCi=10 -6 x. Ci=3. 7 x 104 dis/sec n. Ci=10 -9 x. Ci=3. 7 x 10 dis/sec p. Ci=10 -12 x. Ci=3. 7 x 10 -2 dis/sec Dr. Hanan Y. Abbas- dhmd 70@hotmail. com 3

1 Bq=1 dis/sec. A becquerel is a small unit and defined as the radioactivity of a sample which is decaying at a rate of one disintegration per second. Relationshop between (Bq) and (Ci) 1 Bq=27. 03 x 10 -12 Ci= 27. 03 p. Ci. KBq=103 Bq=27. 03 n. Ci MBq=106 Bq=27. 03 μCi GBq=109 Bq=27. 03 m. Ci Dr. Hanan Y. Abbas- dhmd 70@hotmail. com 4

Law of Decay Rt=λ. Nt (2) Where λ is known as the decay constant, Nt is the number of the radioatoms present at a time t. decay constant λ is defined as the probability of decay per unit time for a single radioatom. λ is a characteristic of a given radionuclide and different for different radionuclides. From Eq. (2) for the different radionuclides (that is different λ), the number of radioatoms (Nt ) that must be present to produce the same amount of radioactivity (Rt)varies. For example , the no of radioatoms present in a sample of 99 m. Tc, which has a radioactivity of 1 m. Ci, is not the same as the no of radioatoms present in a sample of 131 I, which has a radioactivity of 1 m. Ci. Dr. Hanan Y. Abbashanan_abbas 1@windowslive. com 5

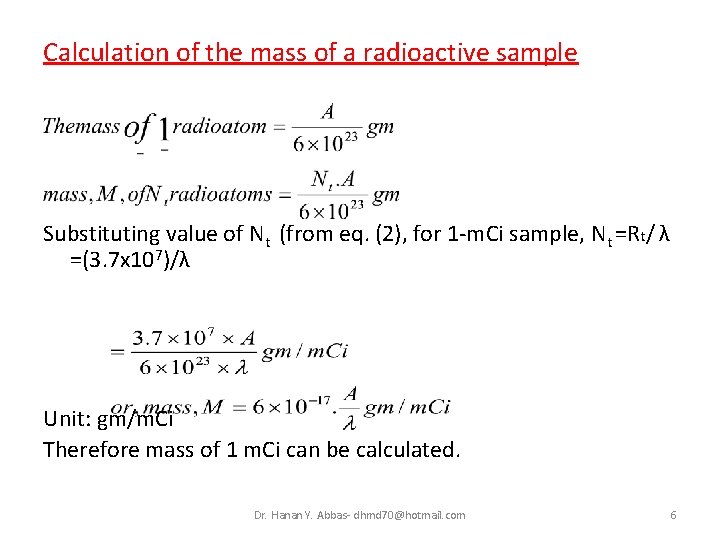
Calculation of the mass of a radioactive sample Substituting value of Nt (from eq. (2), for 1 -m. Ci sample, Nt =Rt/ λ =(3. 7 x 107)/λ Unit: gm/m. Ci Therefore mass of 1 m. Ci can be calculated. Dr. Hanan Y. Abbas- dhmd 70@hotmail. com 6

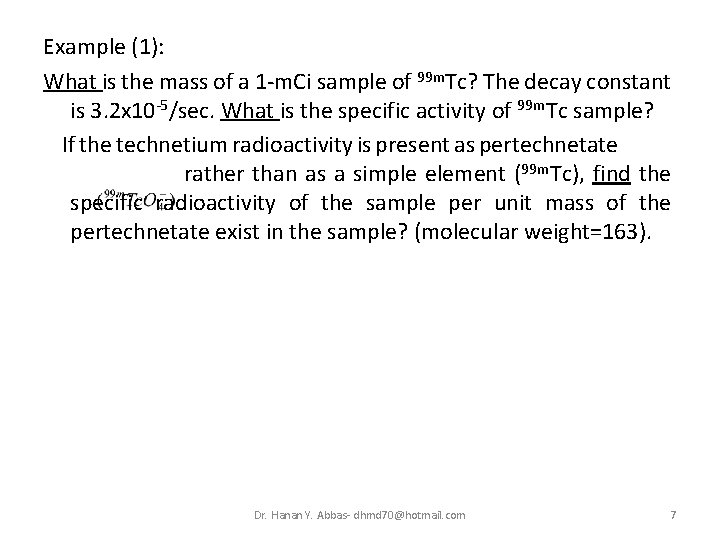
Example (1): What is the mass of a 1 -m. Ci sample of 99 m. Tc? The decay constant is 3. 2 x 10 -5/sec. What is the specific activity of 99 m. Tc sample? If the technetium radioactivity is present as pertechnetate e rather than as a simple element (99 m. Tc), find the specific radioactivity of the sample per unit mass of the pertechnetate exist in the sample? (molecular weight=163). Dr. Hanan Y. Abbas- dhmd 70@hotmail. com 7

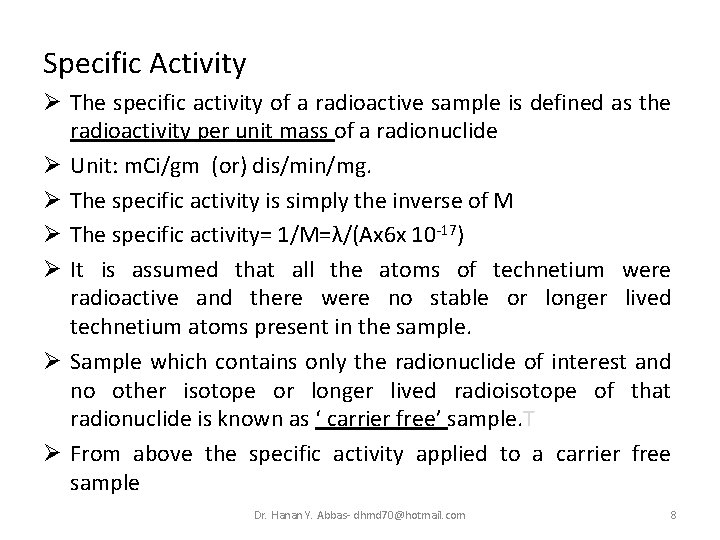
Specific Activity Ø The specific activity of a radioactive sample is defined as the radioactivity per unit mass of a radionuclide Ø Unit: m. Ci/gm (or) dis/min/mg. Ø The specific activity is simply the inverse of M Ø The specific activity= 1/M=λ/(Ax 6 x 10 -17) Ø It is assumed that all the atoms of technetium were radioactive and there were no stable or longer lived technetium atoms present in the sample. Ø Sample which contains only the radionuclide of interest and no other isotope or longer lived radioisotope of that radionuclide is known as ‘ carrier free’ sample. T Ø From above the specific activity applied to a carrier free sample Dr. Hanan Y. Abbas- dhmd 70@hotmail. com 8

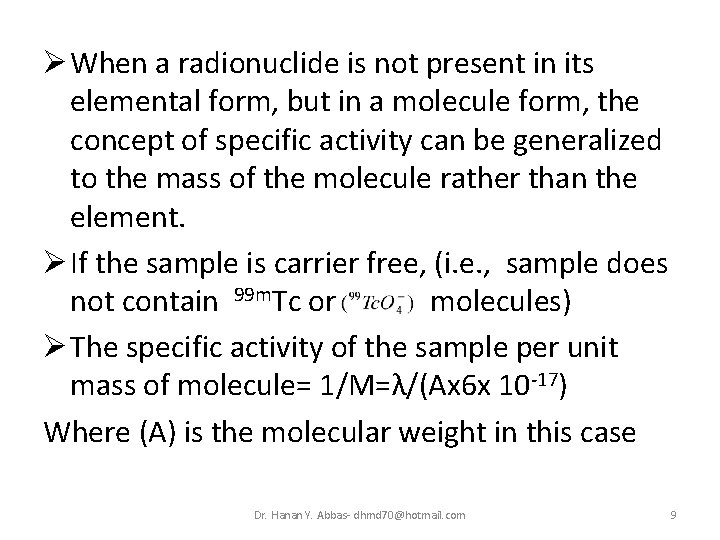
Ø When a radionuclide is not present in its elemental form, but in a molecule form, the concept of specific activity can be generalized to the mass of the molecule rather than the element. Ø If the sample is carrier free, (i. e. , sample does not contain 99 m. Tc or molecules) Ø The specific activity of the sample per unit mass of molecule= 1/M=λ/(Ax 6 x 10 -17) Where (A) is the molecular weight in this case Dr. Hanan Y. Abbas- dhmd 70@hotmail. com 9

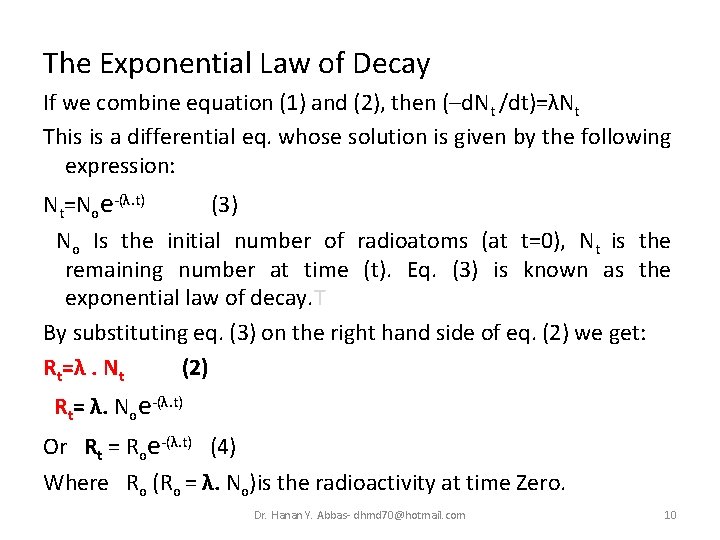
The Exponential Law of Decay If we combine equation (1) and (2), then (–d. Nt /dt)=λNt This is a differential eq. whose solution is given by the following expression: Nt=Noe-(λ. t) (3) No Is the initial number of radioatoms (at t=0), Nt is the remaining number at time (t). Eq. (3) is known as the exponential law of decay. T By substituting eq. (3) on the right hand side of eq. (2) we get: Rt=λ. Nt (2) Rt= λ. Noe-(λ. t) Or Rt = Roe-(λ. t) (4) Where Ro (Ro = λ. No)is the radioactivity at time Zero. Dr. Hanan Y. Abbas- dhmd 70@hotmail. com 10

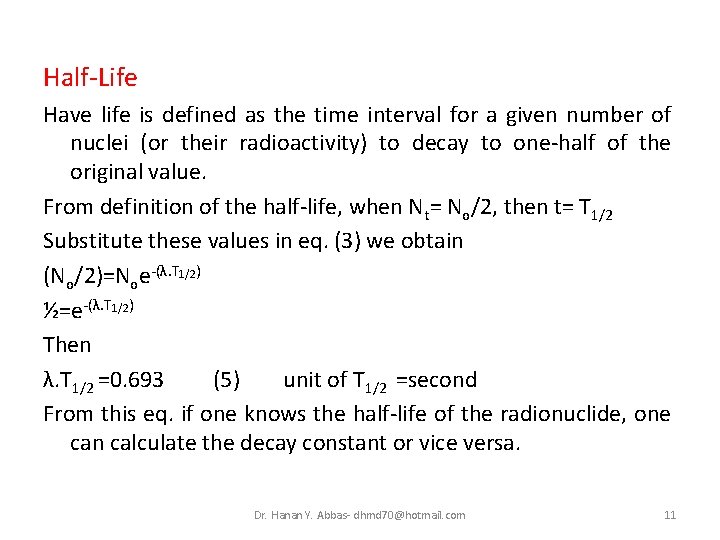
Half-Life Have life is defined as the time interval for a given number of nuclei (or their radioactivity) to decay to one-half of the original value. From definition of the half-life, when Nt= No/2, then t= T 1/2 Substitute these values in eq. (3) we obtain (No/2)=Noe-(λ. T 1/2) ½=e-(λ. T 1/2) Then λ. T 1/2 =0. 693 (5) unit of T 1/2 =second From this eq. if one knows the half-life of the radionuclide, one can calculate the decay constant or vice versa. Dr. Hanan Y. Abbas- dhmd 70@hotmail. com 11

Example (2): Half-life of 131 I=8 days. Determine the decay constant for 131 I. Dr. Hanan Y. Abbas- dhmd 70@hotmail. com 12

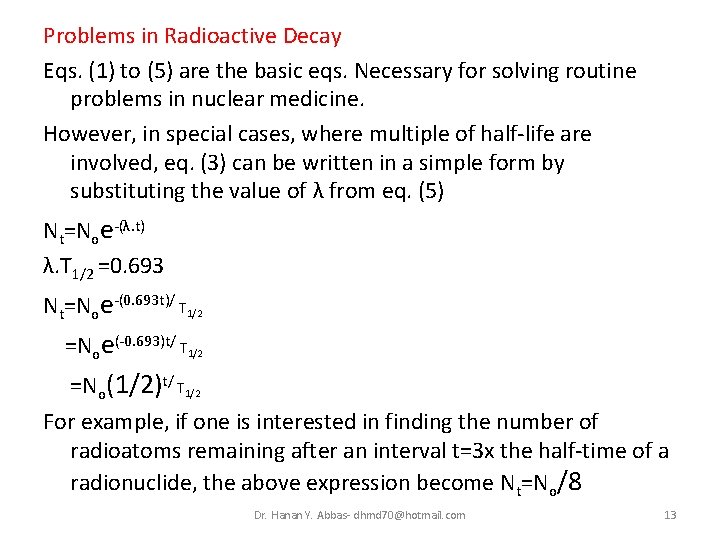
Problems in Radioactive Decay Eqs. (1) to (5) are the basic eqs. Necessary for solving routine problems in nuclear medicine. However, in special cases, where multiple of half-life are involved, eq. (3) can be written in a simple form by substituting the value of λ from eq. (5) Nt=Noe-(λ. t) λ. T 1/2 =0. 693 Nt=Noe-(0. 693 t)/ T 1/2 =Noe(-0. 693)t/ T 1/2 =No(1/2)t/ T 1/2 For example, if one is interested in finding the number of radioatoms remaining after an interval t=3 x the half-time of a radionuclide, the above expression become Nt=No/8 Dr. Hanan Y. Abbas- dhmd 70@hotmail. com 13

Rt =Ro(1/2)t/ T 1/2 (6 b) Where t is time interval. Example (3): A radioactive sample of 99 m. Tc contains 10 -m. Ci radioactivity at 9: 00 A. M What will be the radioactivity of this sample at 3: 00 P. M. on the same day? (The half-life of 99 m. Tc is 6 hr). Dr. Hanan Y. Abbas- dhmd 70@hotmail. com 14

Ø Average Life (Tav) Ø Occasionally we encounter the use of another term known as average life of a radionuclide. It is related to the half-life of the radionuclide or the decay constant λ as follows: Ø Tav=1. 44 x T 1/2=1/ λ Ø Since in radioactive decay not all radioatoms decay at the same time, each individual radioatom exists for a different period of time. Average life is therefore the mean of all these intervals for which individual radioatoms remain in existence Dr. Hanan Y. Abbas- dhmd 70@hotmail. com 15

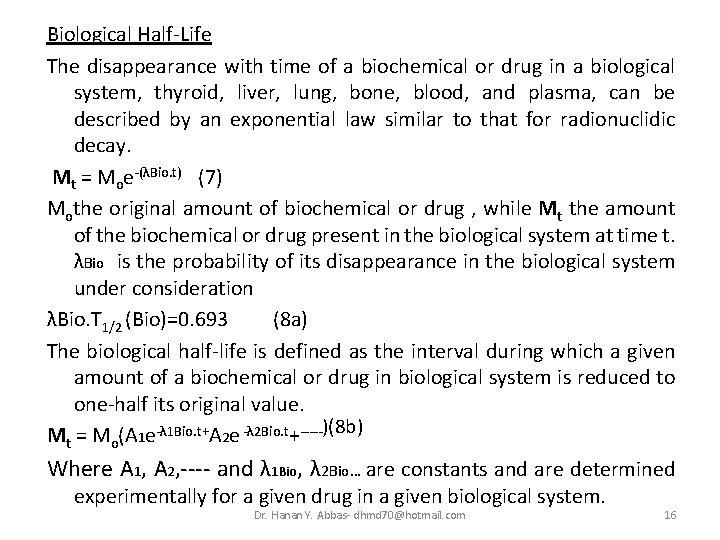
Biological Half-Life The disappearance with time of a biochemical or drug in a biological system, thyroid, liver, lung, bone, blood, and plasma, can be described by an exponential law similar to that for radionuclidic decay. Mt = Moe-(λBio. t) (7) Mothe original amount of biochemical or drug , while Mt the amount of the biochemical or drug present in the biological system at time t. λBio is the probability of its disappearance in the biological system under consideration λBio. T 1/2 (Bio)=0. 693 (8 a) The biological half-life is defined as the interval during which a given amount of a biochemical or drug in biological system is reduced to one-half its original value. Mt = Mo(A 1 e-λ 1 Bio. t+A 2 e -λ 2 Bio. t+-----)(8 b) Where A 1, A 2, ---- and λ 1 Bio, λ 2 Bio… are constants and are determined experimentally for a given drug in a given biological system. Dr. Hanan Y. Abbas- dhmd 70@hotmail. com 16

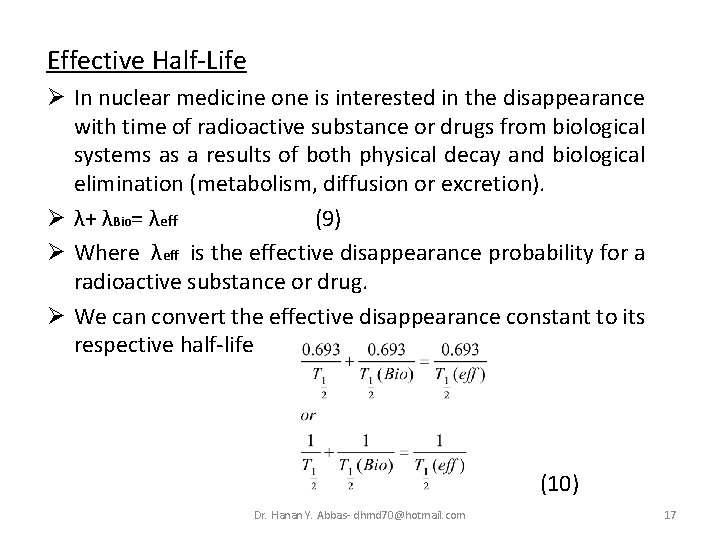
Effective Half-Life Ø In nuclear medicine one is interested in the disappearance with time of radioactive substance or drugs from biological systems as a results of both physical decay and biological elimination (metabolism, diffusion or excretion). Ø λ+ λBio= λeff (9) Ø Where λeff is the effective disappearance probability for a radioactive substance or drug. Ø We can convert the effective disappearance constant to its respective half-life (10) Dr. Hanan Y. Abbas- dhmd 70@hotmail. com 17

Ø From this relationship eq. (10), one can determine the effective half-life of a radiopharmaceutical if the physical halflife of the radionuclide and the biological half-life of the drug (or chemical) are known. Ø However, T 1/2(eff) will always be less than or equal to the shorter of the two T 1/2 and T 1/2 (Bio) Dr. Hanan Y. Abbas- dhmd 70@hotmail. com 18

Example (4): The biological half-life of iodine in human thyroid is about 64 days, and the physical half-life of 131 I is 8 days. Determine the effective half-life of 131 I in the thyroid. Dr. Hanan Y. Abbas- dhmd 70@hotmail. com 19
 Half life equation exponential decay
Half life equation exponential decay How to find the decay factor
How to find the decay factor Beta minus decay vs beta plus decay
Beta minus decay vs beta plus decay Newton's first law and second law and third law
Newton's first law and second law and third law Si unit of newton's first law
Si unit of newton's first law Natural and artificial radioactivity
Natural and artificial radioactivity Natural vs artificial radioactivity
Natural vs artificial radioactivity Natural and artificial radioactivity
Natural and artificial radioactivity Datación radiométrica
Datación radiométrica Key terms radioactivity and nuclear reactions
Key terms radioactivity and nuclear reactions Joy luck club discussion questions by chapter
Joy luck club discussion questions by chapter Parts of clasp
Parts of clasp Multiple clasp
Multiple clasp Who discovered radioactivity
Who discovered radioactivity Who discovered radioactivity
Who discovered radioactivity Who discovered radioactivity
Who discovered radioactivity Natural vs artificial radioactivity
Natural vs artificial radioactivity Radioactivity as spontaneous disintegration
Radioactivity as spontaneous disintegration Radioactivity
Radioactivity Radioactivity
Radioactivity