Radioactivity II Alpha Beta Radiation Assoc Prof RNDr

Radioactivity II Alpha – Beta Radiation Assoc. Prof. RNDr. Mgr. Katarína Kozlíková, CSc. IMPh. BPh. ITM FM CU in Bratislava katarina. kozlikova@fmed. uniba. sk © K. Kozlíková, 2020 Radioactivity II - Alpha - Beta Radiation 1

Contents l l Introduction Alpha decay l l l Beta decay l l l Interaction of alpha radiation with matter Linear energy transfer Range, energy spectrum and path of an alpha particle Protection against alpha radiation Types of beta decay Interaction of beta radiation with matter Range, energy spectrum and path of a beta particle Protection against beta radiation Literature © K. Kozlíková, 2020 Radioactivity II - Alpha - Beta Radiation 2

Introduction l l Radioactive isotopes Some atoms (isotopes) are unstable l l Radioactive decay represents spontaneous decay of nuclei, while l l l Their nuclei decay New nuclei are created The energy state of the nucleus changes At the radioactive decay a nucleus emits at least one particle © K. Kozlíková, 2020 Radioactivity II - Alpha - Beta Radiation 3
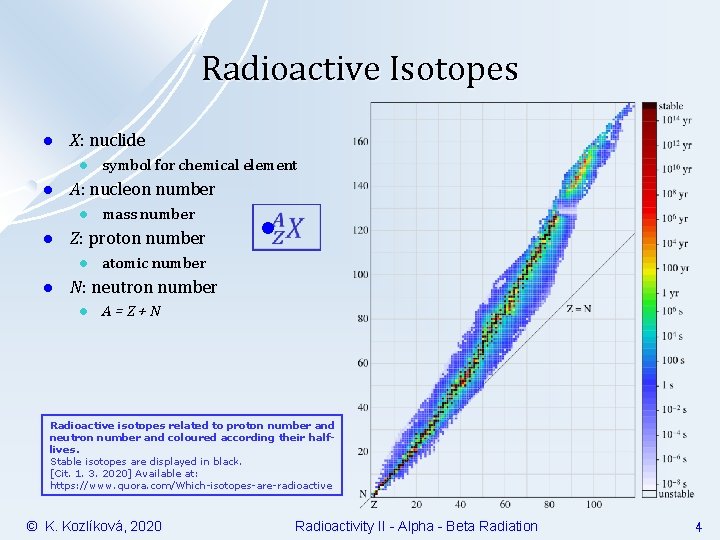
Radioactive Isotopes l X: nuclide l l A: nucleon number l l mass number Z: proton number l l symbol for chemical element l atomic number N: neutron number l A=Z+N Radioactive isotopes related to proton number and neutron number and coloured according their halflives. Stable isotopes are displayed in black. [Cit. 1. 3. 2020] Available at: https: //www. quora. com/Which-isotopes-are-radioactive © K. Kozlíková, 2020 Radioactivity II - Alpha - Beta Radiation 4
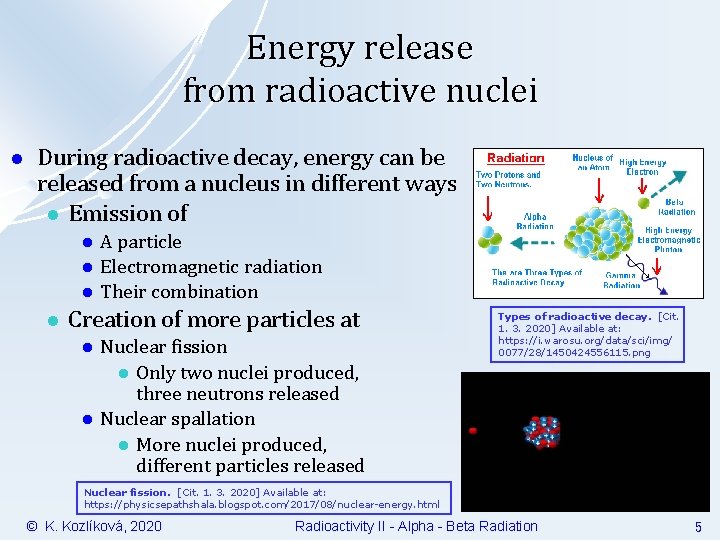
Energy release from radioactive nuclei l During radioactive decay, energy can be released from a nucleus in different ways l Emission of l l A particle Electromagnetic radiation Their combination Creation of more particles at l l Nuclear fission l Only two nuclei produced, three neutrons released Nuclear spallation l More nuclei produced, different particles released Types of radioactive decay. [Cit. 1. 3. 2020] Available at: https: //i. warosu. org/data/sci/img/ 0077/28/1450424556115. png Nuclear fission. [Cit. 1. 3. 2020] Available at: https: //physicsepathshala. blogspot. com/2017/08/nuclear-energy. html © K. Kozlíková, 2020 Radioactivity II - Alpha - Beta Radiation 5

Alpha decay © K. Kozlíková, 2020 Radioactivity II - Alpha - Beta Radiation 6
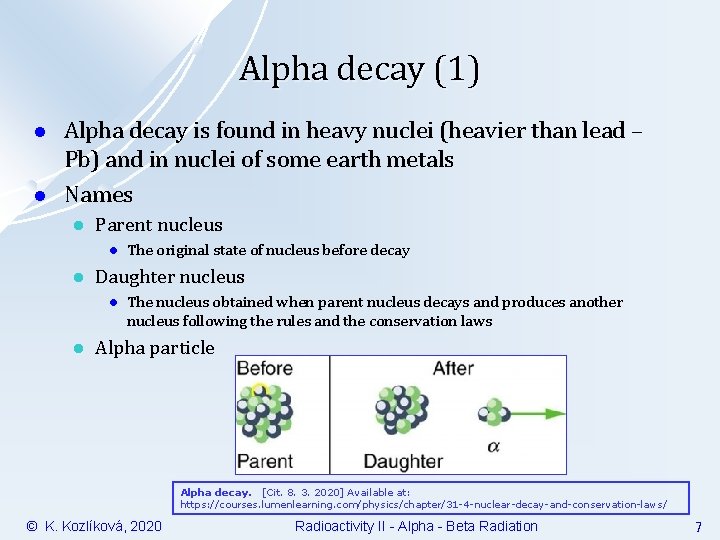
Alpha decay (1) l l Alpha decay is found in heavy nuclei (heavier than lead – Pb) and in nuclei of some earth metals Names l Parent nucleus l l Daughter nucleus l l The original state of nucleus before decay The nucleus obtained when parent nucleus decays and produces another nucleus following the rules and the conservation laws Alpha particle Alpha decay. [Cit. 8. 3. 2020] Available at: https: //courses. lumenlearning. com/physics/chapter/31 -4 -nuclear-decay-and-conservation-laws/ © K. Kozlíková, 2020 Radioactivity II - Alpha - Beta Radiation 7
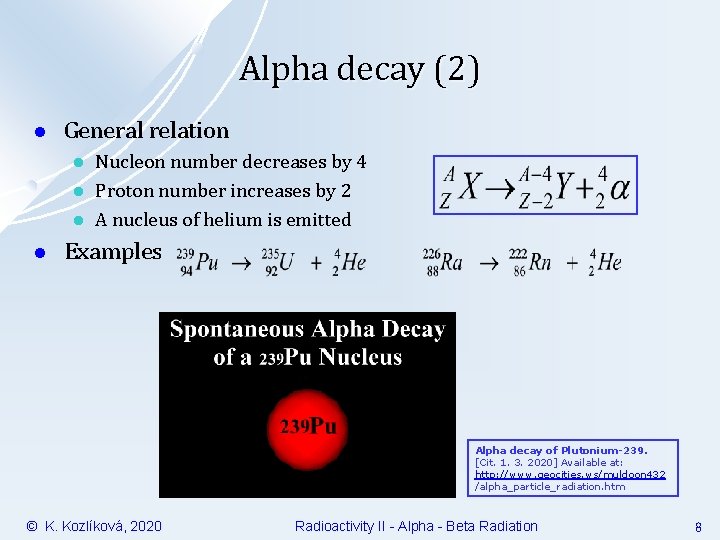
Alpha decay (2) l General relation l l Nucleon number decreases by 4 Proton number increases by 2 A nucleus of helium is emitted Examples Alpha decay of Plutonium-239. [Cit. 1. 3. 2020] Available at: http: //www. geocities. ws/muldoon 432 /alpha_particle_radiation. htm © K. Kozlíková, 2020 Radioactivity II - Alpha - Beta Radiation 8
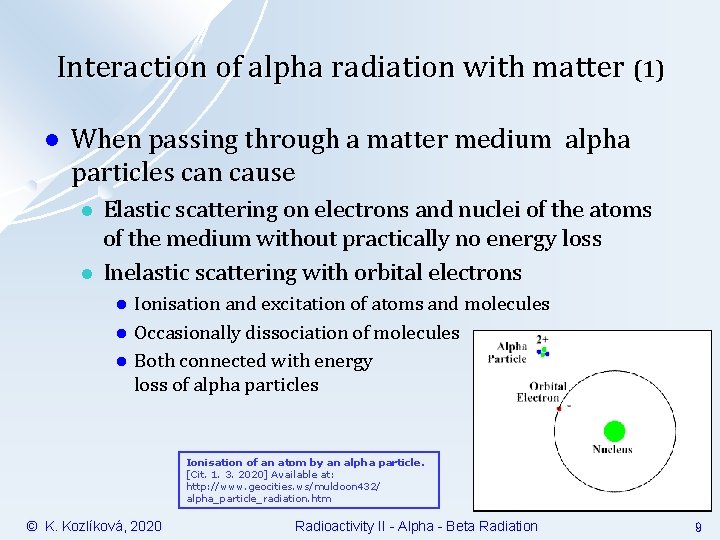
Interaction of alpha radiation with matter (1) l When passing through a matter medium alpha particles can cause l l Elastic scattering on electrons and nuclei of the atoms of the medium without practically no energy loss Inelastic scattering with orbital electrons l l l Ionisation and excitation of atoms and molecules Occasionally dissociation of molecules Both connected with energy loss of alpha particles Ionisation of an atom by an alpha particle. [Cit. 1. 3. 2020] Available at: http: //www. geocities. ws/muldoon 432/ alpha_particle_radiation. htm © K. Kozlíková, 2020 Radioactivity II - Alpha - Beta Radiation 9
Interaction of alpha radiation with matter (2) l Initial velocity v, with which the particle is emitted from the nucleus, corresponds to its energy E l l l v 106 m s-1 E Me. V Energy loss due to ionisation and excitation of the atoms of the medium Ionising power of the medium is given by the specific ionisation l Number of produced ion pairs (n) per unit track length (l) = n / l © K. Kozlíková, 2020 Radioactivity II - Alpha - Beta Radiation 10

Specific ionisation of alpha particles (1) l Specific ionisation in general l l Equals the number of produced ion pairs n per unit length of charged particle´s path l (= n / l) Increases with electrical charge of the particle Decreases with incident particle velocity Specific ionisation of an alpha particle in air l 20 000 pairs - 80 000 pairs / 1 cm An alpha particle detected in an isopropanol cloud chamber. [Cit. 1. 3. 2020] Available at: https: //en. wikipedia. org/wiki/Radiation © K. Kozlíková, 2020 Radioactivity II - Alpha - Beta Radiation 11
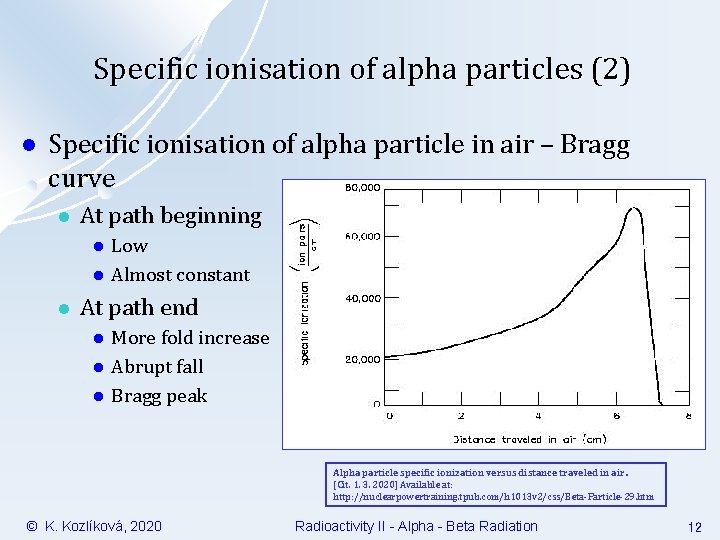
Specific ionisation of alpha particles (2) l Specific ionisation of alpha particle in air – Bragg curve l At path beginning l l l Low Almost constant At path end l l l More fold increase Abrupt fall Bragg peak Alpha particle specific ionization versus distance traveled in air. [Cit. 1. 3. 2020] Available at: http: //nuclearpowertraining. tpub. com/h 1013 v 2/css/Beta-Particle-29. htm © K. Kozlíková, 2020 Radioactivity II - Alpha - Beta Radiation 12
Linear energy transfer (1) l l Expresses how much energy an ionising particle transfers to the medium through which it passes Is used in dosimetry l l Depends on l l l Important factor in assessing potential tissue and organ damage from exposure to ionising radiation The nature of radiation The medium (material) through which the radiation passes Restricted linear energy transfer L∆ l d. E∆ l dx l energy loss of the charged particle due to electronic collisions travelled distance All secondary electrons with kinetic energy Ek > ∆ are excluded © K. Kozlíková, 2020 Radioactivity II - Alpha - Beta Radiation 13
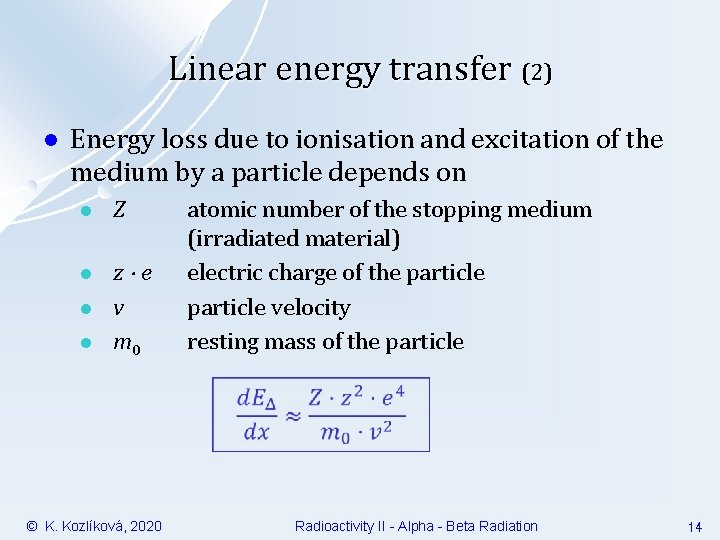
Linear energy transfer (2) l Energy loss due to ionisation and excitation of the medium by a particle depends on l Z l z e v m 0 l l © K. Kozlíková, 2020 atomic number of the stopping medium (irradiated material) electric charge of the particle velocity resting mass of the particle Radioactivity II - Alpha - Beta Radiation 14

Linear energy transfer (LET) and stopping power l If Δ ∞ l l No electrons with larger energy Restricted LET unrestricted LET linear electronic stopping power l l l SI unit: N (newton) Used units: ke. V/μm, Me. V/cm Typical values of LET l l 5 Me. V alpha particles: 100 ke. V/μm Diagnostic X-rays: 3 ke. V/μm © K. Kozlíková, 2020 Radioactivity II - Alpha - Beta Radiation 15

Stopping power of the medium l l Loss of kinetic energy of an ionising particle when passing through a material of some density Energy loss of the particle per unit path length l Calculated as linear ion density times the average energy required to produce 1 pair of ions l Average energy per 1 ion pair in air: ~ 34 e. V l Example l Total number of ion pairs n, which can be created by an alpha particle with energy 6. 8 Me. V © K. Kozlíková, 2020 Radioactivity II - Alpha - Beta Radiation 16
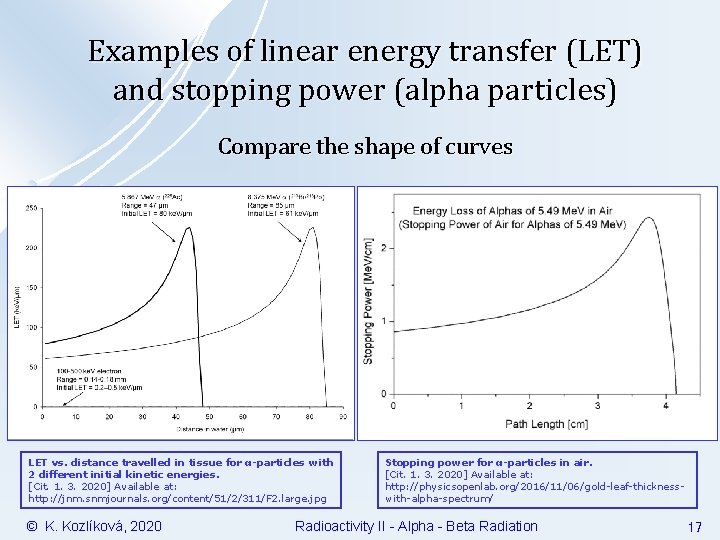
Examples of linear energy transfer (LET) and stopping power (alpha particles) Compare the shape of curves LET vs. distance travelled in tissue for α-particles with 2 different initial kinetic energies. [Cit. 1. 3. 2020] Available at: http: //jnm. snmjournals. org/content/51/2/311/F 2. large. jpg © K. Kozlíková, 2020 Stopping power for α-particles in air. [Cit. 1. 3. 2020] Available at: http: //physicsopenlab. org/2016/11/06/gold-leaf-thicknesswith-alpha-spectrum/ Radioactivity II - Alpha - Beta Radiation 17
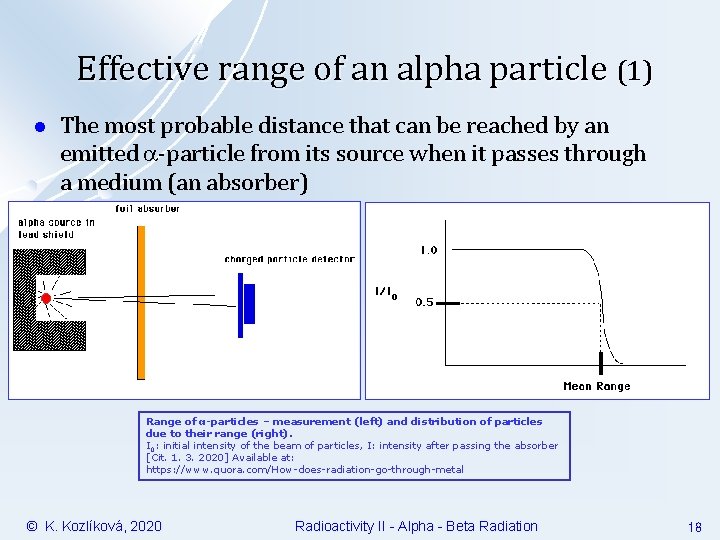
Effective range of an alpha particle (1) l The most probable distance that can be reached by an emitted -particle from its source when it passes through a medium (an absorber) Range of α-particles – measurement (left) and distribution of particles due to their range (right). I 0: initial intensity of the beam of particles, I: intensity after passing the absorber [Cit. 1. 3. 2020] Available at: https: //www. quora. com/How-does-radiation-go-through-metal © K. Kozlíková, 2020 Radioactivity II - Alpha - Beta Radiation 18
![Effective range of an alpha particle (2) l l Effective range R [cm] of Effective range of an alpha particle (2) l l Effective range R [cm] of](http://slidetodoc.com/presentation_image_h2/5ab04a7ea221a31fb0808525e523c336/image-19.jpg)
Effective range of an alpha particle (2) l l Effective range R [cm] of a particle depends on l Energy E [Me. V], with which the particle is emitted l Medium density [g/cm 3] l Nucleon number A of the medium In air l l R ≈ 2 cm to 10 cm R [cm] ≈ 0. 325 · E 3/2 [Me. V] for energies 4 Me. V – 8 Me. V In liquids, soft tissues l R ~ 10 - 100 m In aluminium l Cherry, S. R. , Sorenson, J. A. , & Phelps, M. E. (2012). Interaction of Radiation with Matter. Physics in Nuclear Medicine, 63– 85. doi: 10. 1016/b 978 -1 -4160 -5198 -5. 00006 -x About the half distance corresponding to the energy for liquids © K. Kozlíková, 2020 Radioactivity II - Alpha - Beta Radiation 19
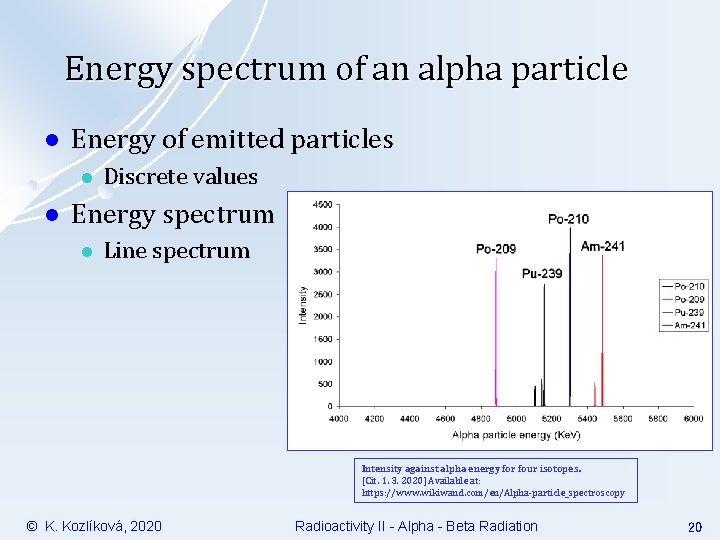
Energy spectrum of an alpha particle l Energy of emitted particles l l Discrete values Energy spectrum l Line spectrum Intensity against alpha energy for four isotopes. [Cit. 1. 3. 2020] Available at: https: //www. wikiwand. com/en/Alpha-particle_spectroscopy © K. Kozlíková, 2020 Radioactivity II - Alpha - Beta Radiation 20
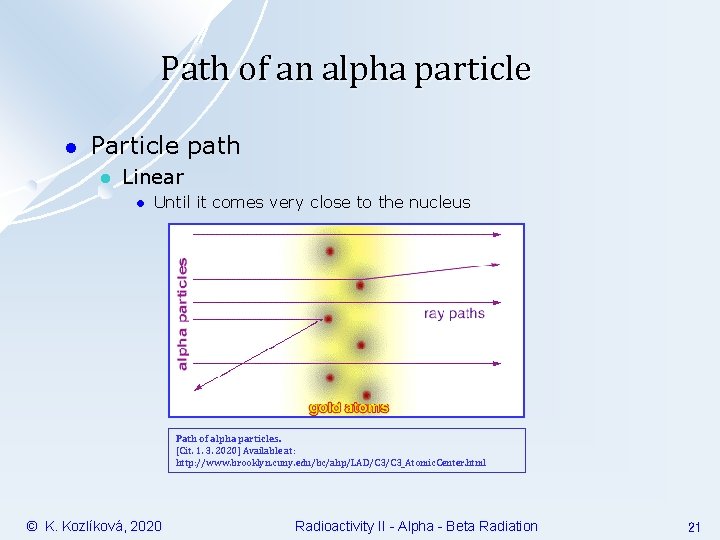
Path of an alpha particle l Particle path l Linear l Until it comes very close to the nucleus Path of alpha particles. [Cit. 1. 3. 2020] Available at: http: //www. brooklyn. cuny. edu/bc/ahp/LAD/C 3_Atomic. Center. html © K. Kozlíková, 2020 Radioactivity II - Alpha - Beta Radiation 21
Processes due to interaction of an alpha particle with medium l l l Production of characteristic X-rays Luminescence of material Chemical processes l l l Cause functional and morphological changes of tissues Transformation of energy into heat Transformation of a nucleus l Interaction of an -particle with a nucleus l Rare © K. Kozlíková, 2020 Radioactivity II - Alpha - Beta Radiation 22

Protection against alpha radiation l Alpha radiation is most dangerous l l l At internal irradiation (internal contamination) after inhalation or ingestion At irradiation of eyes All alpha radiation is absorbed by l l l Surface skin layer Sheet of paper Air layer thicker than 10 cm Penetration of different types of radiation. [Cit. 1. 3. 2020] Available at: https: //www. researchgate. net/figure/Figure-22 -Penetrationpower-of-ionizing-radiation-40_fig 6_307466518 © K. Kozlíková, 2020 Radioactivity II - Alpha - Beta Radiation 23

Beta decay © K. Kozlíková, 2020 Radioactivity II - Alpha - Beta Radiation 24
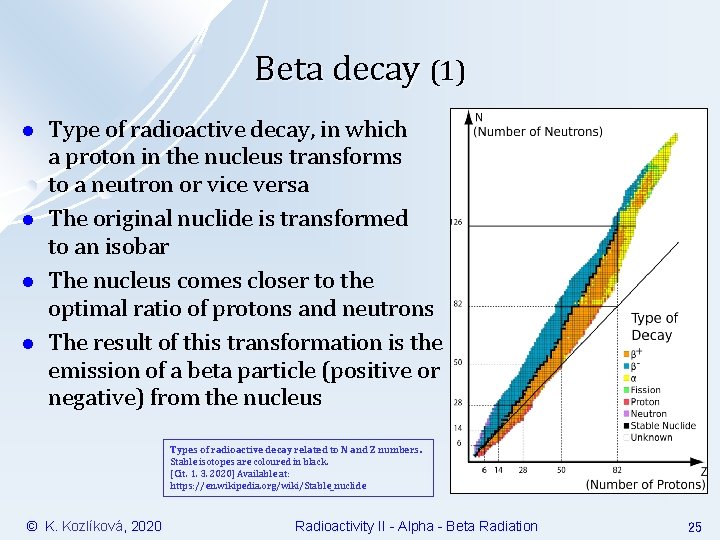
Beta decay (1) l l Type of radioactive decay, in which a proton in the nucleus transforms to a neutron or vice versa The original nuclide is transformed to an isobar The nucleus comes closer to the optimal ratio of protons and neutrons The result of this transformation is the emission of a beta particle (positive or negative) from the nucleus Types of radioactive decay related to N and Z numbers. Stable isotopes are coloured in black. [Cit. 1. 3. 2020] Available at: https: //en. wikipedia. org/wiki/Stable_nuclide © K. Kozlíková, 2020 Radioactivity II - Alpha - Beta Radiation 25

Beta decay (2) l l Beta particles are high energy electrons l They are emitted from an unstable nucleus l They have elementary electric charge negative or positive l Positively charged particle beta is a positron l Negatively charged particle beta is an electron (negatron) The majority of radionuclides used in biomedical research are beta emitters © K. Kozlíková, 2020 Radioactivity II - Alpha - Beta Radiation 26
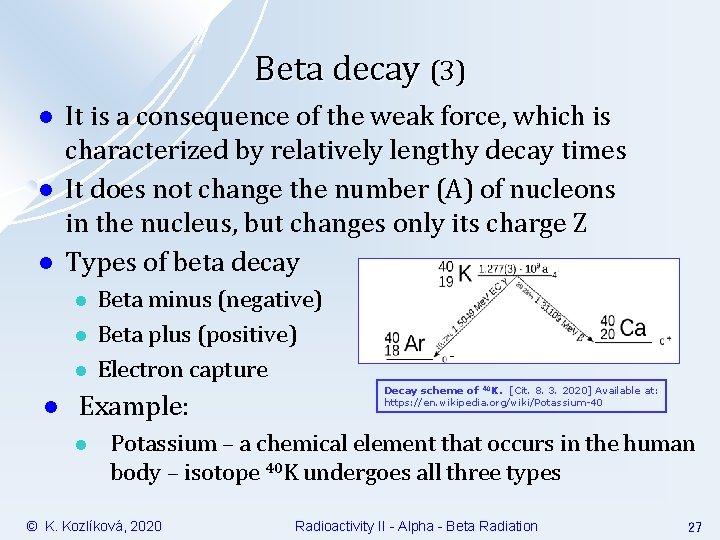
Beta decay (3) l l l It is a consequence of the weak force, which is characterized by relatively lengthy decay times It does not change the number (A) of nucleons in the nucleus, but changes only its charge Z Types of beta decay l l Beta minus (negative) Beta plus (positive) Electron capture Example: l Decay scheme of 40 K. [Cit. 8. 3. 2020] Available at: https: //en. wikipedia. org/wiki/Potassium-40 Potassium – a chemical element that occurs in the human body – isotope 40 K undergoes all three types © K. Kozlíková, 2020 Radioactivity II - Alpha - Beta Radiation 27
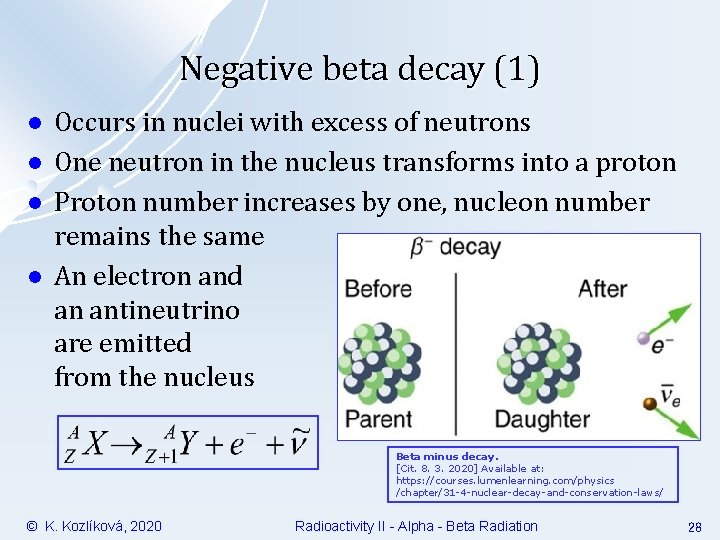
Negative beta decay (1) l l Occurs in nuclei with excess of neutrons One neutron in the nucleus transforms into a proton Proton number increases by one, nucleon number remains the same An electron and an antineutrino are emitted from the nucleus Beta minus decay. [Cit. 8. 3. 2020] Available at: https: //courses. lumenlearning. com/physics /chapter/31 -4 -nuclear-decay-and-conservation-laws/ © K. Kozlíková, 2020 Radioactivity II - Alpha - Beta Radiation 28
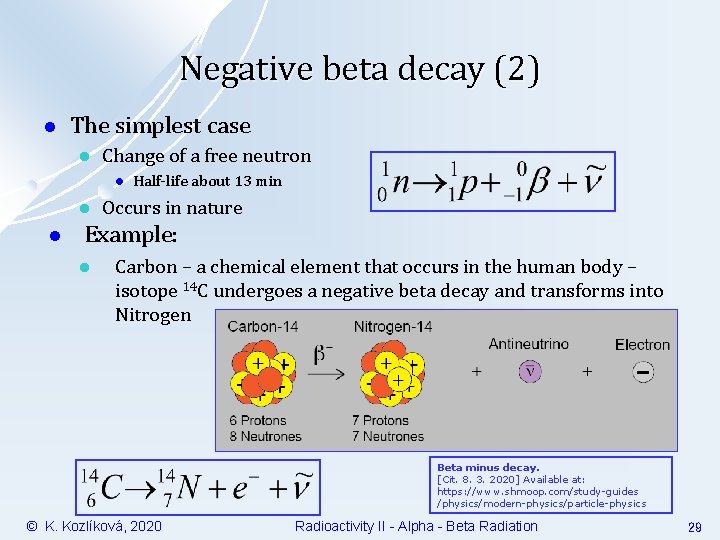
Negative beta decay (2) l The simplest case l Change of a free neutron l l l Half-life about 13 min Occurs in nature Example: l Carbon – a chemical element that occurs in the human body – isotope 14 C undergoes a negative beta decay and transforms into Nitrogen Beta minus decay. [Cit. 8. 3. 2020] Available at: https: //www. shmoop. com/study-guides /physics/modern-physics/particle-physics © K. Kozlíková, 2020 Radioactivity II - Alpha - Beta Radiation 29
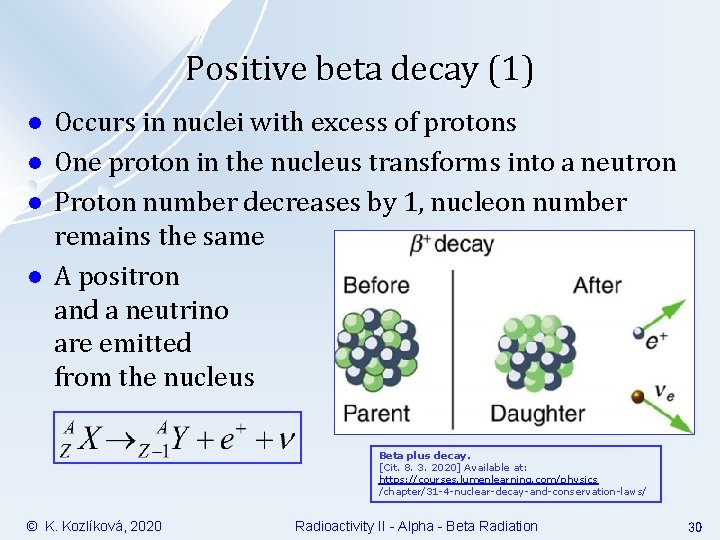
Positive beta decay (1) l l Occurs in nuclei with excess of protons One proton in the nucleus transforms into a neutron Proton number decreases by 1, nucleon number remains the same A positron and a neutrino are emitted from the nucleus Beta plus decay. [Cit. 8. 3. 2020] Available at: https: //courses. lumenlearning. com/physics /chapter/31 -4 -nuclear-decay-and-conservation-laws/ © K. Kozlíková, 2020 Radioactivity II - Alpha - Beta Radiation 30
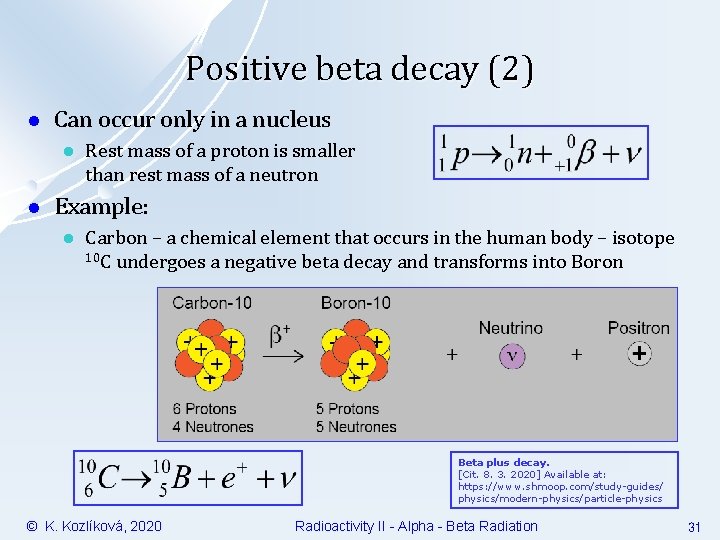
Positive beta decay (2) l Can occur only in a nucleus l l Rest mass of a proton is smaller than rest mass of a neutron Example: l Carbon – a chemical element that occurs in the human body – isotope 10 C undergoes a negative beta decay and transforms into Boron Beta plus decay. [Cit. 8. 3. 2020] Available at: https: //www. shmoop. com/study-guides/ physics/modern-physics/particle-physics © K. Kozlíková, 2020 Radioactivity II - Alpha - Beta Radiation 31
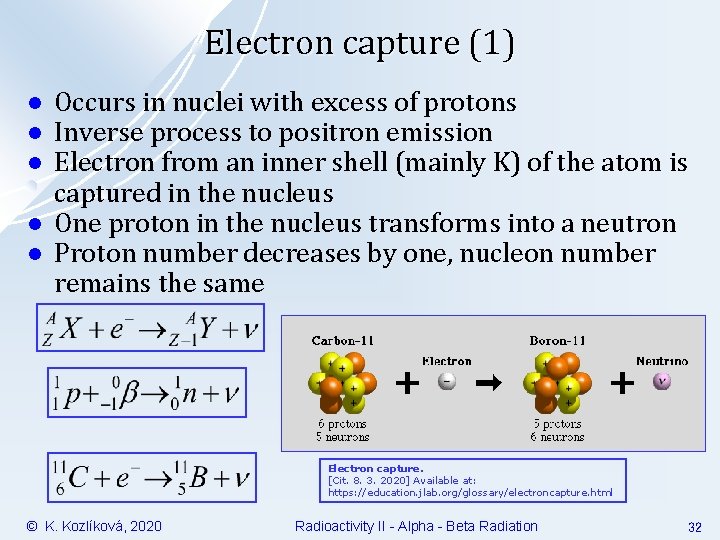
Electron capture (1) l l l Occurs in nuclei with excess of protons Inverse process to positron emission Electron from an inner shell (mainly K) of the atom is captured in the nucleus One proton in the nucleus transforms into a neutron Proton number decreases by one, nucleon number remains the same Electron capture. [Cit. 8. 3. 2020] Available at: https: //education. jlab. org/glossary/electroncapture. html © K. Kozlíková, 2020 Radioactivity II - Alpha - Beta Radiation 32
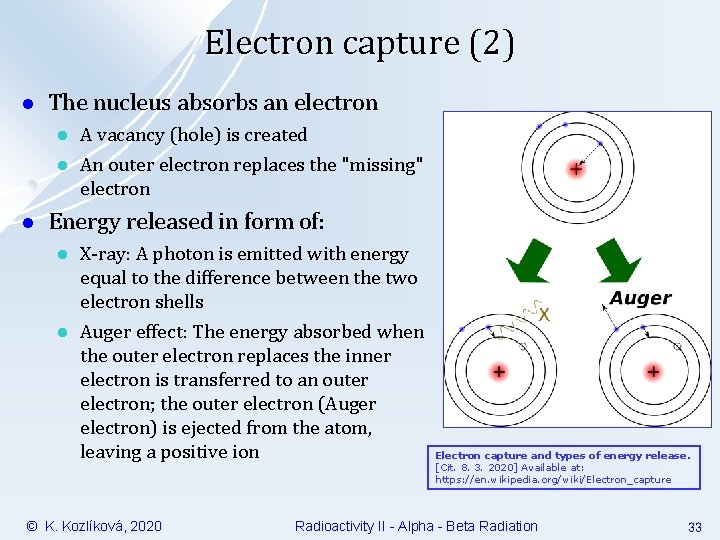
Electron capture (2) l The nucleus absorbs an electron l l l A vacancy (hole) is created An outer electron replaces the "missing" electron Energy released in form of: l l X-ray: A photon is emitted with energy equal to the difference between the two electron shells Auger effect: The energy absorbed when the outer electron replaces the inner electron is transferred to an outer electron; the outer electron (Auger electron) is ejected from the atom, leaving a positive ion © K. Kozlíková, 2020 Electron capture and types of energy release. [Cit. 8. 3. 2020] Available at: https: //en. wikipedia. org/wiki/Electron_capture Radioactivity II - Alpha - Beta Radiation 33
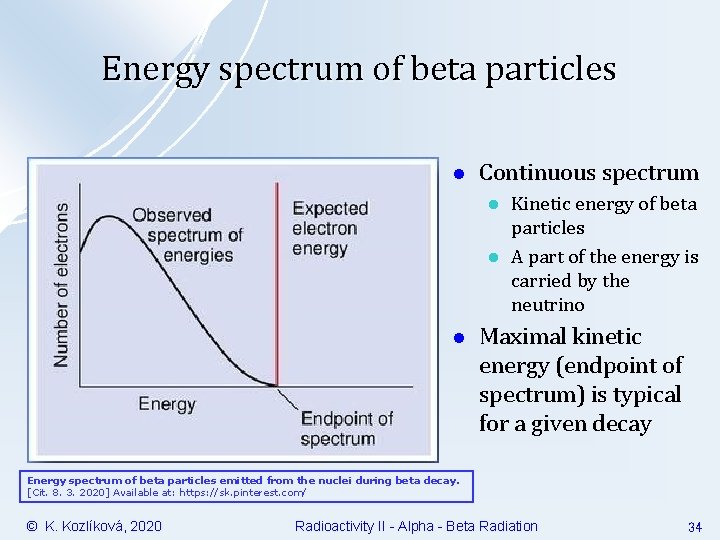
Energy spectrum of beta particles l Continuous spectrum l l l Kinetic energy of beta particles A part of the energy is carried by the neutrino Maximal kinetic energy (endpoint of spectrum) is typical for a given decay Energy spectrum of beta particles emitted from the nuclei during beta decay. [Cit. 8. 3. 2020] Available at: https: //sk. pinterest. com/ © K. Kozlíková, 2020 Radioactivity II - Alpha - Beta Radiation 34
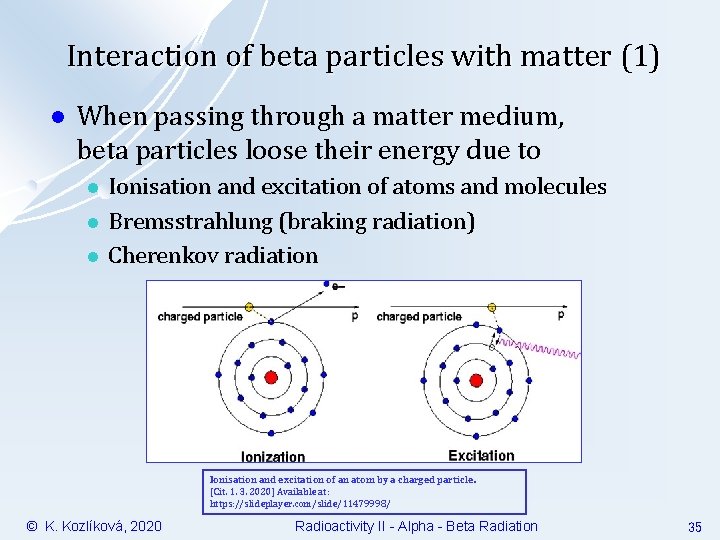
Interaction of beta particles with matter (1) l When passing through a matter medium, beta particles loose their energy due to l l l Ionisation and excitation of atoms and molecules Bremsstrahlung (braking radiation) Cherenkov radiation Ionisation and excitation of an atom by a charged particle. [Cit. 1. 3. 2020] Available at: https: //slideplayer. com/slide/11479998/ © K. Kozlíková, 2020 Radioactivity II - Alpha - Beta Radiation 35
Interaction of beta particles with matter (2) l Initial velocity v, with which the particle is emitted from the nucleus l l v 108 m s-1 (v c) Relativistic mass of the particle Ionisation of medium produced by an electron. [Cit. 1. 3. 2020] Available at: http: //www. sprawls. org/ppmi 2/INTERACT/ © K. Kozlíková, 2020 Radioactivity II - Alpha - Beta Radiation 36
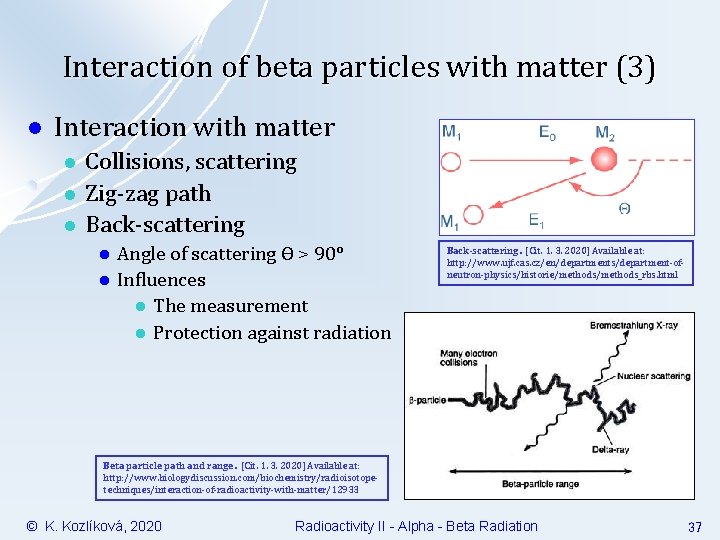
Interaction of beta particles with matter (3) l Interaction with matter l l l Collisions, scattering Zig-zag path Back-scattering l l Angle of scattering Ɵ > 90° Influences l The measurement l Protection against radiation Back-scattering. [Cit. 1. 3. 2020] Available at: http: //www. ujf. cas. cz/en/departments/department-ofneutron-physics/historie/methods_rbs. html Beta particle path and range. [Cit. 1. 3. 2020] Available at: http: //www. biologydiscussion. com/biochemistry/radioisotopetechniques/interaction-of-radioactivity-with-matter/12933 © K. Kozlíková, 2020 Radioactivity II - Alpha - Beta Radiation 37
![Effective range of beta particles l Effective range R [mg/cm 2] depends on l Effective range of beta particles l Effective range R [mg/cm 2] depends on l](http://slidetodoc.com/presentation_image_h2/5ab04a7ea221a31fb0808525e523c336/image-38.jpg)
Effective range of beta particles l Effective range R [mg/cm 2] depends on l l In air l l R ~ 0. 1 m – 1 m In liquids, soft tissues l l Energy E [Me. V], with which the particle is emitted R 1 cm Path of the particle is about 4 -times longer than its range Range-energy curves for beta rays. [Cit. 1. 3. 2020] Available at: https: //courses. ecampus. oregonstate. edu/ne 581/three/index 3. htm © K. Kozlíková, 2020 Radioactivity II - Alpha - Beta Radiation 38
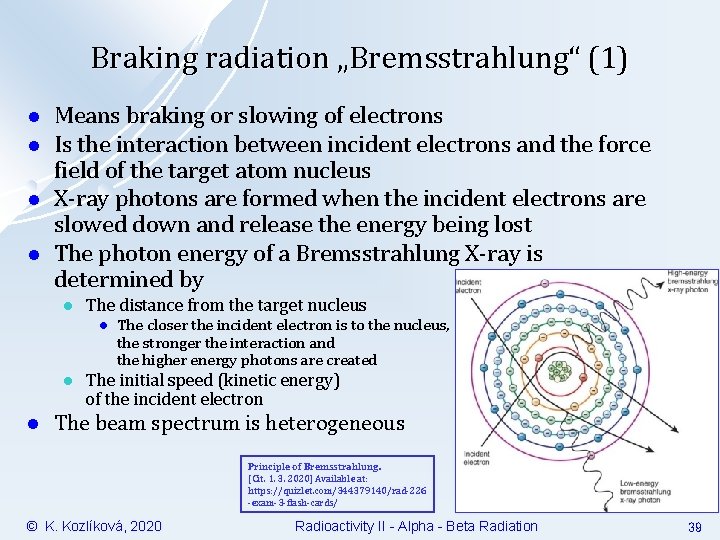
Braking radiation „Bremsstrahlung“ (1) l l Means braking or slowing of electrons Is the interaction between incident electrons and the force field of the target atom nucleus X-ray photons are formed when the incident electrons are slowed down and release the energy being lost The photon energy of a Bremsstrahlung X-ray is determined by l The distance from the target nucleus l l l The closer the incident electron is to the nucleus, the stronger the interaction and the higher energy photons are created The initial speed (kinetic energy) of the incident electron The beam spectrum is heterogeneous Principle of Bremsstrahlung. [Cit. 1. 3. 2020] Available at: https: //quizlet. com/344379140/rad-226 -exam-3 -flash-cards/ © K. Kozlíková, 2020 Radioactivity II - Alpha - Beta Radiation 39
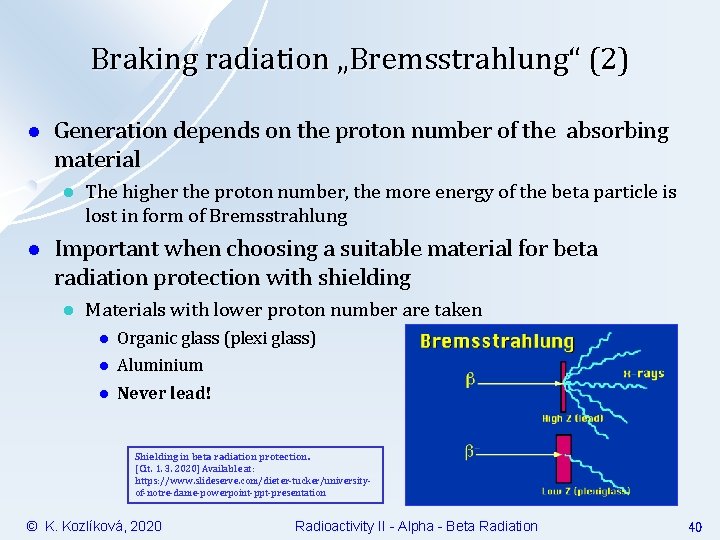
Braking radiation „Bremsstrahlung“ (2) l Generation depends on the proton number of the absorbing material l l The higher the proton number, the more energy of the beta particle is lost in form of Bremsstrahlung Important when choosing a suitable material for beta radiation protection with shielding l Materials with lower proton number are taken l Organic glass (plexi glass) l Aluminium l Never lead! Shielding in beta radiation protection. [Cit. 1. 3. 2020] Available at: https: //www. slideserve. com/dieter-tucker/universityof-notre-dame-powerpoint-ppt-presentation © K. Kozlíková, 2020 Radioactivity II - Alpha - Beta Radiation 40
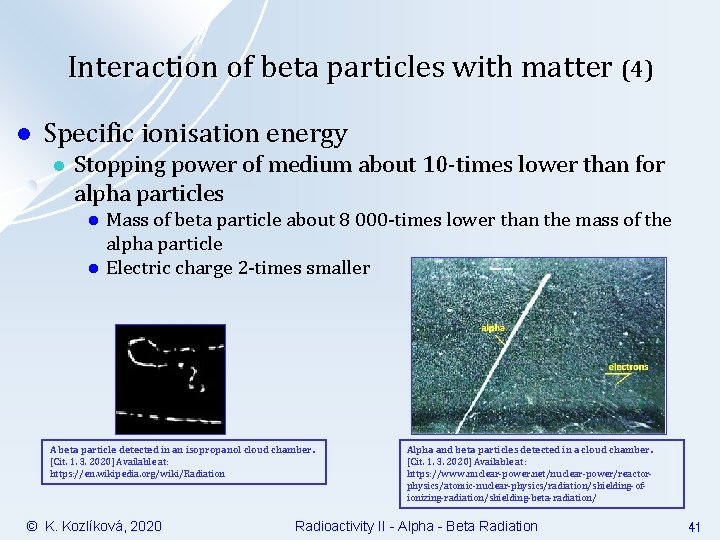
Interaction of beta particles with matter (4) l Specific ionisation energy l Stopping power of medium about 10 -times lower than for alpha particles l l Mass of beta particle about 8 000 -times lower than the mass of the alpha particle Electric charge 2 -times smaller A beta particle detected in an isopropanol cloud chamber. [Cit. 1. 3. 2020] Available at: https: //en. wikipedia. org/wiki/Radiation © K. Kozlíková, 2020 Alpha and beta particles detected in a cloud chamber. [Cit. 1. 3. 2020] Available at: https: //www. nuclear-power. net/nuclear-power/reactorphysics/atomic-nuclear-physics/radiation/shielding-ofionizing-radiation/shielding-beta-radiation/ Radioactivity II - Alpha - Beta Radiation 41
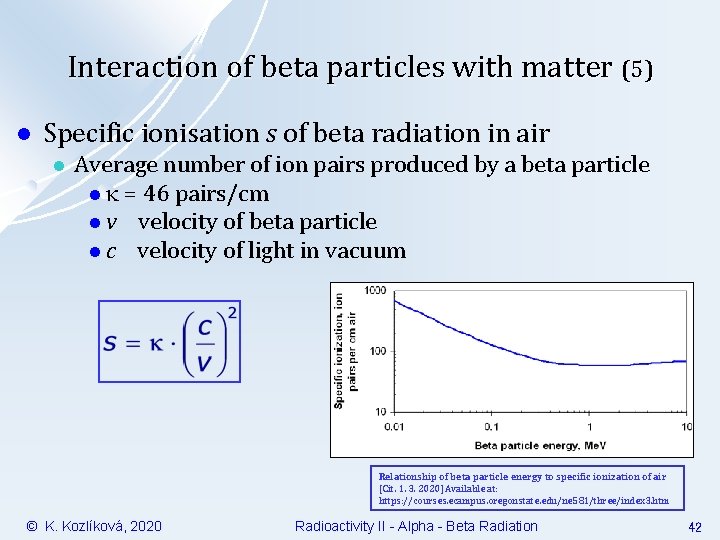
Interaction of beta particles with matter (5) l Specific ionisation s of beta radiation in air l Average number of ion pairs produced by a beta particle l = 46 pairs/cm l v velocity of beta particle l c velocity of light in vacuum Relationship of beta particle energy to specific ionization of air [Cit. 1. 3. 2020] Available at: https: //courses. ecampus. oregonstate. edu/ne 581/three/index 3. htm © K. Kozlíková, 2020 Radioactivity II - Alpha - Beta Radiation 42

Cherenkov radiation l If the velocity of the flying β particle is larger than the velocity of light in the given medium l l Analogous to a sonic boom Photons of electromagnetic radiation with wavelengths between ultraviolet and visible light are produced Appears in nuclear reactors Can be used to detect beta particles Cherenkov radiation caused by beta particles. [Cit. 8. 3. 2020] Available at: https: //reactor. mst. edu/cerenkov/ © K. Kozlíková, 2020 Radioactivity II - Alpha - Beta Radiation 43
Processes due to interaction of a beta particle with medium l Effects l l l Chemical Photochemical Biological Electron (left) and positron (right) interaction with matter. [Cit. 8. 3. 2020] Available at: http: //www. geocities. ws/muldoon 432/beta_particle_radiation. htm Emission of characteristic X-rays Bremsstrahlung l Deflection from the original direction of the particle path in the electric field of the nucleus © K. Kozlíková, 2020 Radioactivity II - Alpha - Beta Radiation 44
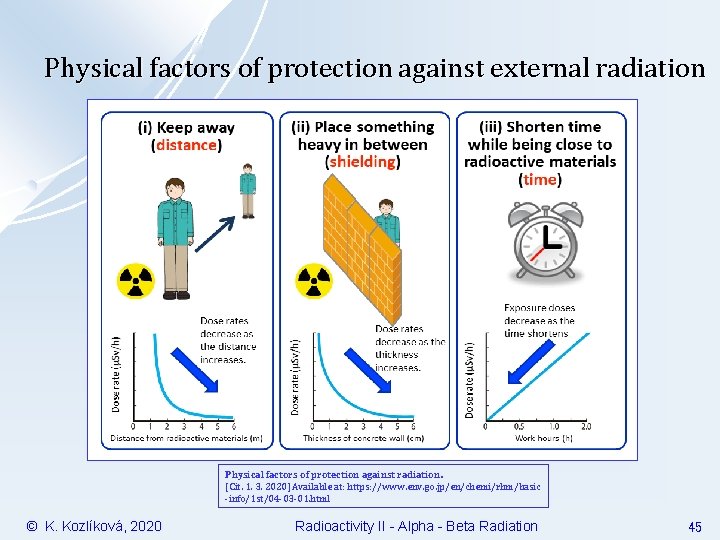
Physical factors of protection against external radiation Physical factors of protection against radiation. [Cit. 1. 3. 2020] Available at: https: //www. env. go. jp/en/chemi/rhm/basic -info/1 st/04 -03 -01. html © K. Kozlíková, 2020 Radioactivity II - Alpha - Beta Radiation 45
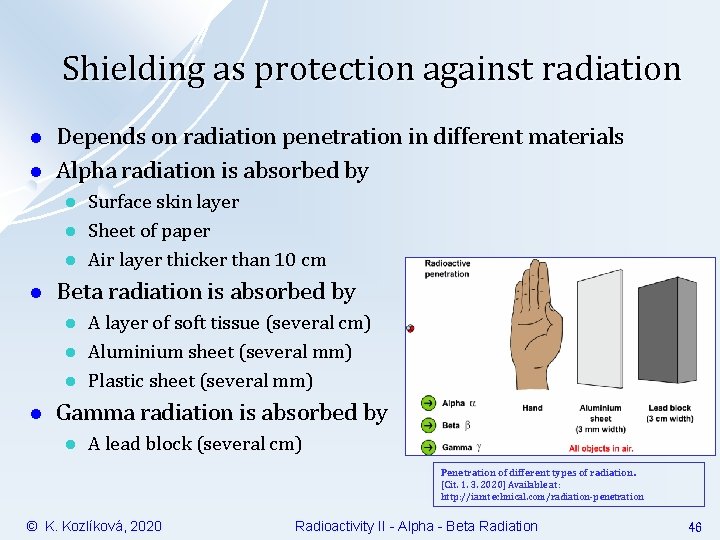
Shielding as protection against radiation l l Depends on radiation penetration in different materials Alpha radiation is absorbed by l l Beta radiation is absorbed by l l Surface skin layer Sheet of paper Air layer thicker than 10 cm A layer of soft tissue (several cm) Aluminium sheet (several mm) Plastic sheet (several mm) Gamma radiation is absorbed by l A lead block (several cm) Penetration of different types of radiation. [Cit. 1. 3. 2020] Available at: http: //iamtechnical. com/radiation-penetration © K. Kozlíková, 2020 Radioactivity II - Alpha - Beta Radiation 46
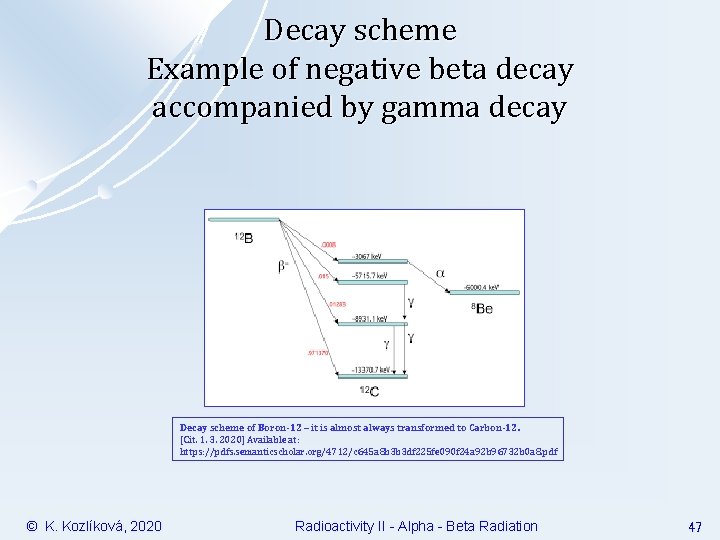
Decay scheme Example of negative beta decay accompanied by gamma decay Decay scheme of Boron-12 – it is almost always transformed to Carbon-12. [Cit. 1. 3. 2020] Available at: https: //pdfs. semanticscholar. org/4712/c 645 a 8 b 3 b 3 df 225 fe 090 f 24 a 92 b 96732 b 0 a 8. pdf © K. Kozlíková, 2020 Radioactivity II - Alpha - Beta Radiation 47
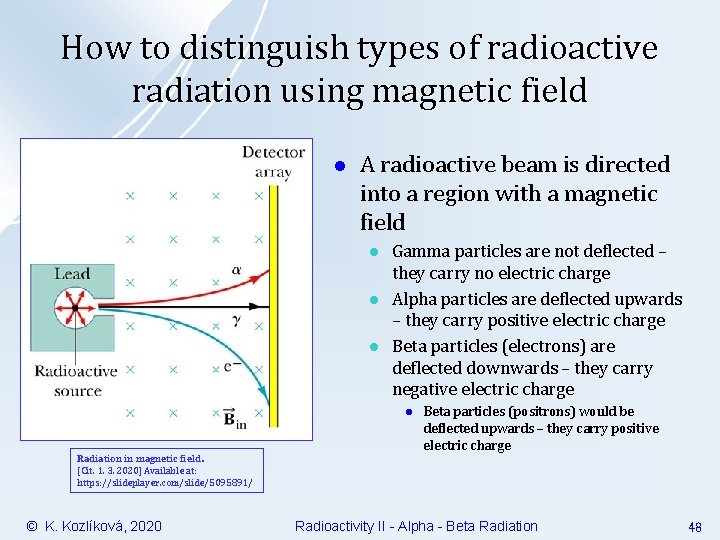
How to distinguish types of radioactive radiation using magnetic field l A radioactive beam is directed into a region with a magnetic field l l l Gamma particles are not deflected – they carry no electric charge Alpha particles are deflected upwards – they carry positive electric charge Beta particles (electrons) are deflected downwards – they carry negative electric charge l Radiation in magnetic field. [Cit. 1. 3. 2020] Available at: https: //slideplayer. com/slide/5095891/ © K. Kozlíková, 2020 Beta particles (positrons) would be deflected upwards – they carry positive electric charge Radioactivity II - Alpha - Beta Radiation 48

Literature l ALLISY-ROBERTS, P. , WILLIAMS, J. Farr’s Physics for Medical Imaging. Edinburgh : Saunders - Elsevier, 2008. 207 p. ISBN 978 -0 -7020 -2844 -1 l HRAZDIRA, I. , MORNSTEIN, V. , BOUREK, A. , ŠKORPÍKOVÁ, J. Fundamentals of Biophysics and Medical Technology. 2 nd revised edition. Brno : Masaryk University, Faculty of Medicine, 2012. 325 p. ISBN 978 -80 -210 -5758 -6. JIRÁK, D. , VÍTEK, F. Basics of Medical Physics. Praha : Charles University, Karolinum Press, 2017. 223 p. ISBN 978 -80 -246 -3810 -2. l l KOZLÍKOVÁ, K. , MARTINKA, J. Theory And Tasks For Practicals On Medical Biophysics. Brno : Librix, 2010. 248 p. ISBN 978 -80 -7399 -881 -3 l RONTÓ, G. , TARJÁN, I. (eds. ) An Introduction To Biophysics With Medical Orientation. Budapest : Akadémiai Kiadó, 1997. 447 p. ISBN 963 -05 -7607 -4. STN EN ISO 80000 -10: Veličiny a jednotky. Časť 10: Atómová a jadrová fyzika (ISO 80000 -10: 2009). Bratislava : Úrad pre normalizáciu, metrológiu a skúšobníctvo SR, 2017. 80 s. Electronic sources listed directly in the text. l l © K. Kozlíková, 2020 Radioactivity II - Alpha - Beta Radiation 49
- Slides: 49