Radioactivity Henri Becquerel put some uranium crystals on

Radioactivity
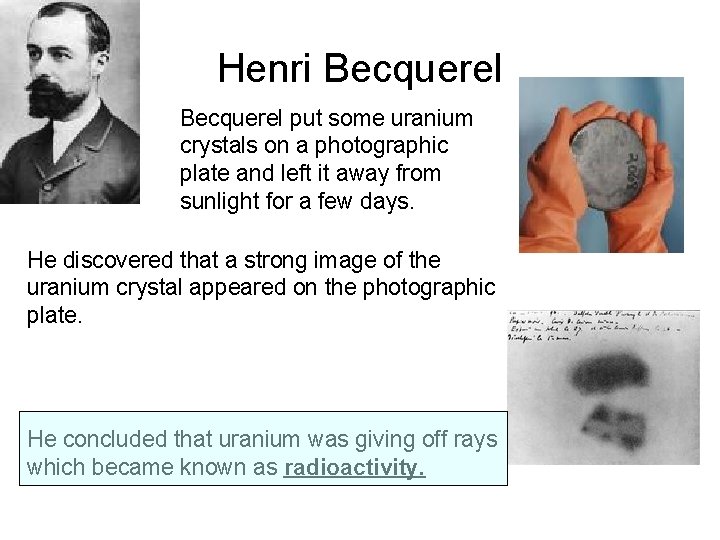
Henri Becquerel put some uranium crystals on a photographic plate and left it away from sunlight for a few days. He discovered that a strong image of the uranium crystal appeared on the photographic plate. He concluded that uranium was giving off rays which became known as radioactivity.

The mystery of Pitchblende ore Becquerel could not understand why Pitchblende ore gave off such a high amount of radiation. Although it did contain uranium it still was giving off more radiation than even pure uranium gave off!

Marie Curie, and her husband Pierre, found that Pitchblende ore not only contained Uranium but also contained two new elements which she called Radium and Polonium are both more radioactive than Uranium

Nobel Prizes • 1903 - The Nobel Prize in Physics Becquerel and the Curies shared the Nobel prize for the “discovery of spontaneous radioactivity“ and “joint research on the radiation phenomena discovered by Professor Henri Becquerel” • 1911 - Curie was the first female to win a Nobel prize in Chemistry for her research on radium and Polonium. Radiation damage to researchers… Marie Curie (66) died from leukaemia in 1934 Becquerel (55) died from radiation burns in 1908
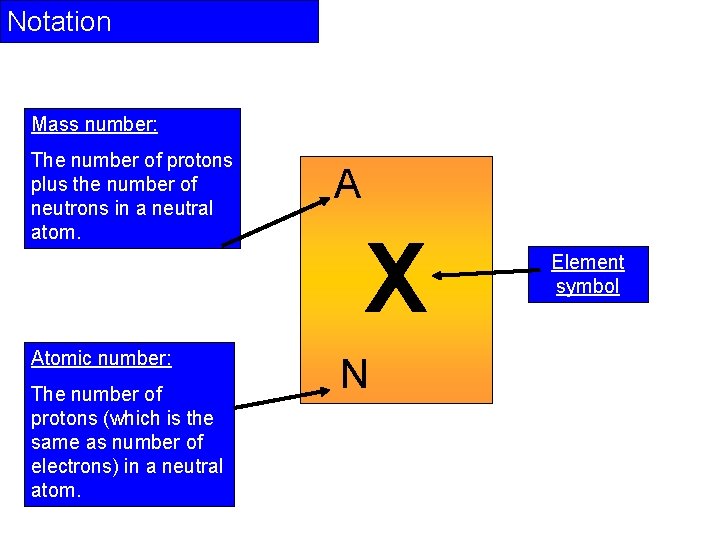
Notation Mass number: The number of protons plus the number of neutrons in a neutral atom. Atomic number: The number of protons (which is the same as number of electrons) in a neutral atom. A X N Element symbol
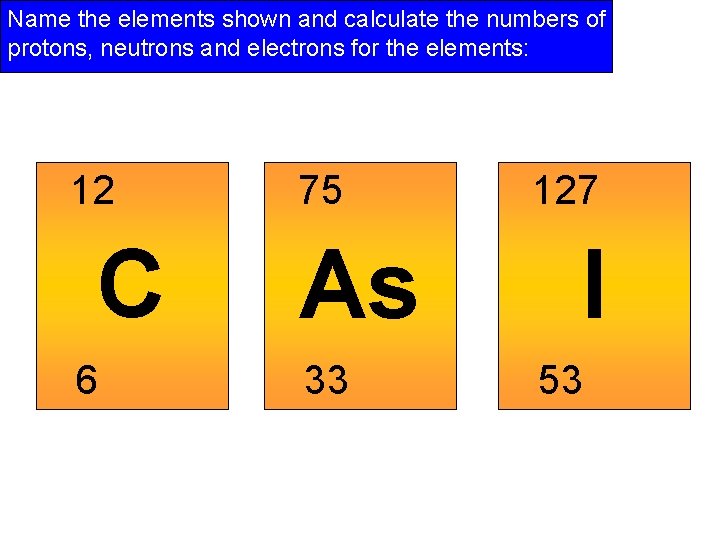
Name the elements shown and calculate the numbers of protons, neutrons and electrons for the elements: 12 C 6 75 As 33 127 I 53
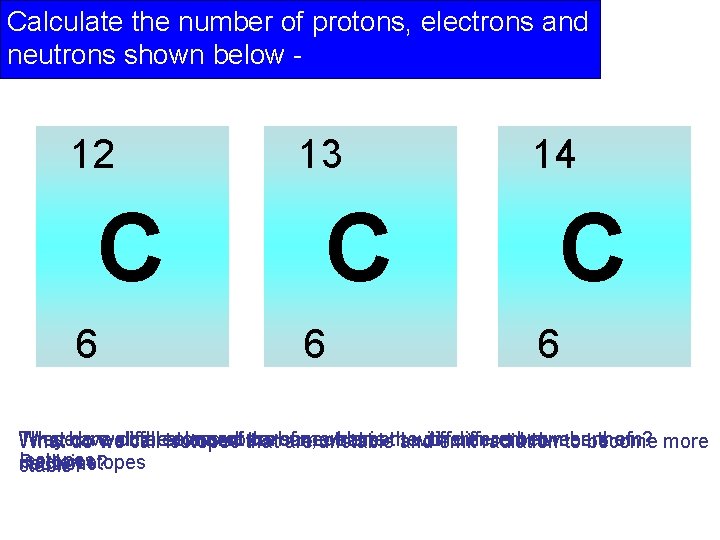
Calculate the number of protons, electrons and neutrons shown below - 12 C 6 13 C 6 14 C 6 Thesehave They are different theisotopes atoms element numbers of that the carbon, of same neutrons. what element is the with difference different between numbers them? of What do weall call are unstable and emit radiation to become more Isotopes Radioisotopes neutrons? stable?
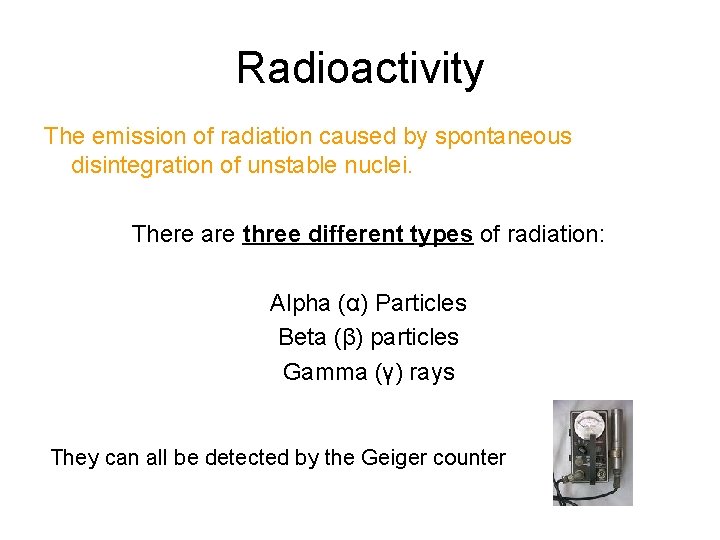
Radioactivity The emission of radiation caused by spontaneous disintegration of unstable nuclei. There are three different types of radiation: Alpha (α) Particles Beta (β) particles Gamma (γ) rays They can all be detected by the Geiger counter
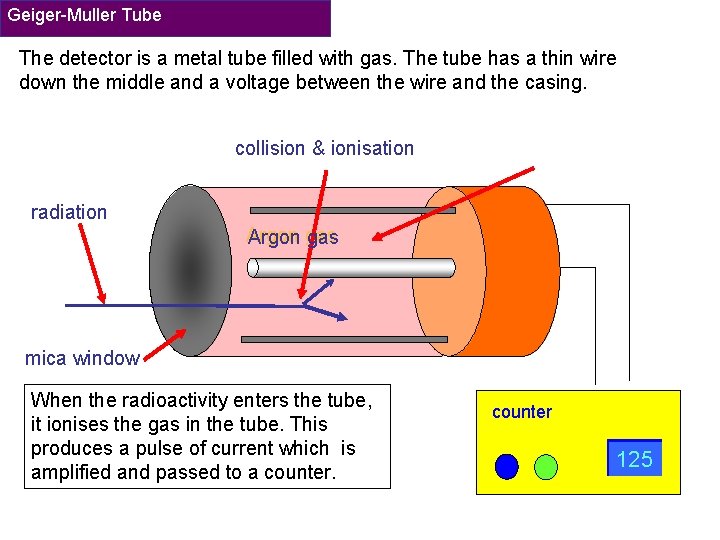
Geiger-Muller Tube The detector is a metal tube filled with gas. The tube has a thin wire down the middle and a voltage between the wire and the casing. collision & ionisation radiation Argon gas mica window When the radioactivity enters the tube, it ionises the gas in the tube. This produces a pulse of current which is amplified and passed to a counter 124 125
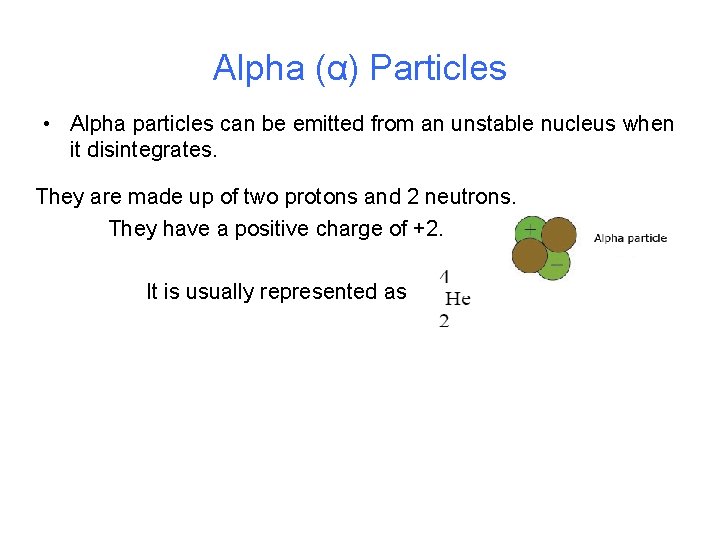
Alpha (α) Particles • Alpha particles can be emitted from an unstable nucleus when it disintegrates. They are made up of two protons and 2 neutrons. They have a positive charge of +2. It is usually represented as
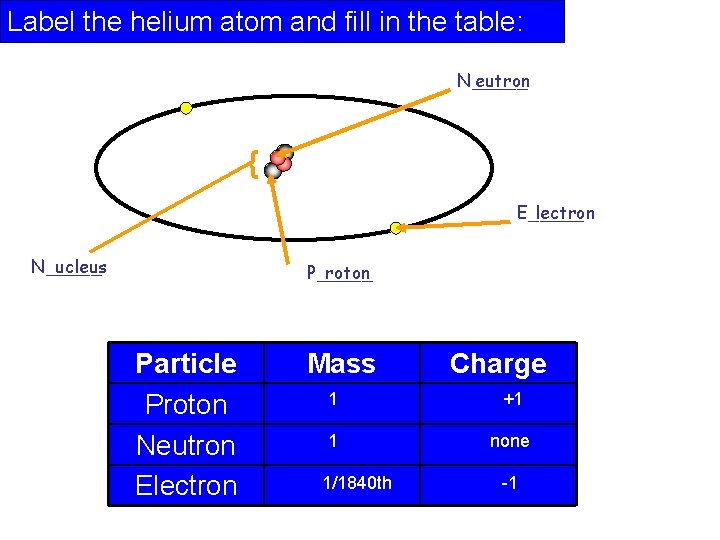
Label the helium atom and fill in the table: N_____ eutron { E_____ lectron N_____ ucleus P_____ roton Particle Proton Neutron Electron Mass Charge 1 +1 1 none 1/1840 th -1

What we used to think… It was believed that atoms were: 1. Spheres of positive charge. 2. With negative charges spread through it. This resembled a plum-pudding, so it was called the ‘Plum –pudding’ model. This was wrong! How did we discover current ideas about the structure of the atom?
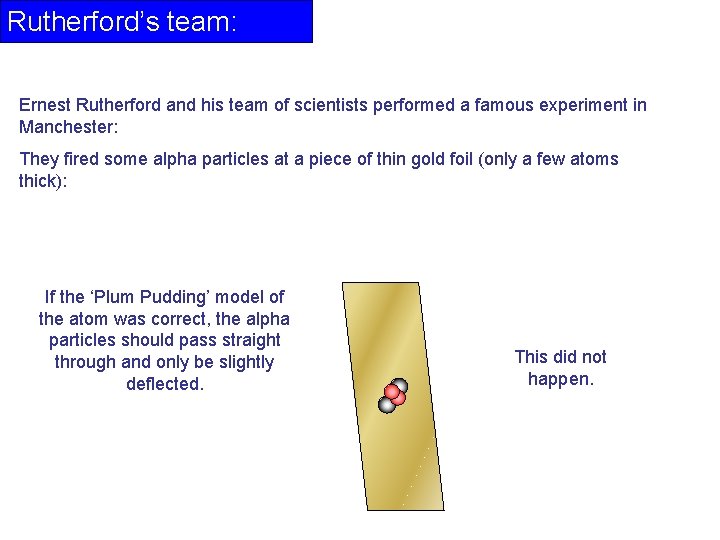
Rutherford’s team: Ernest Rutherford and his team of scientists performed a famous experiment in Manchester: They fired some alpha particles at a piece of thin gold foil (only a few atoms thick): If the ‘Plum Pudding’ model of the atom was correct, the alpha particles should pass straight through and only be slightly deflected. This did not happen.
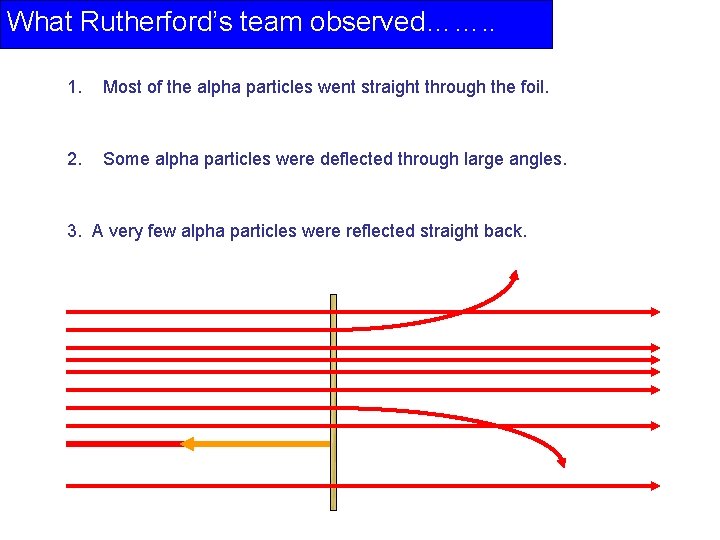
What Rutherford’s team observed……. . 1. Most of the alpha particles went straight through the foil. 2. Some alpha particles were deflected through large angles. 3. A very few alpha particles were reflected straight back.
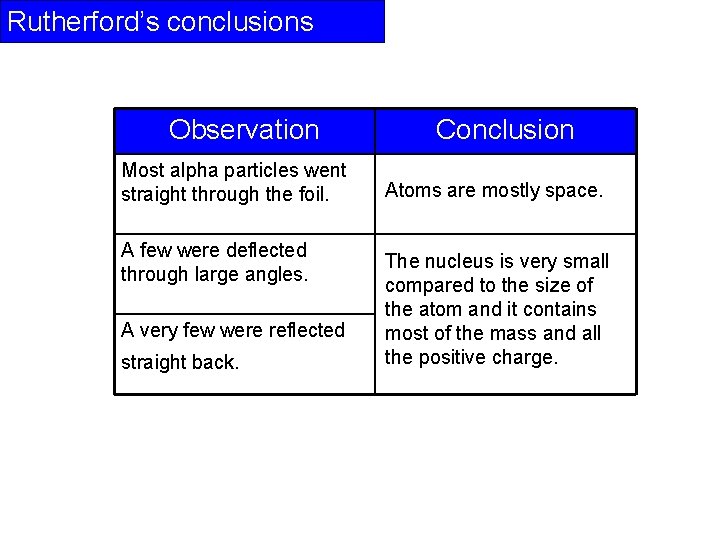
Rutherford’s conclusions Observation Most alpha particles went straight through the foil. A few were deflected through large angles. A very few were reflected straight back. Conclusion Atoms are mostly space. The nucleus is very small compared to the size of the atom and it contains most of the mass and all the positive charge.

Americium-241 is a radioactive isotope that emits alpha particles. Americium-241 is found in smoke detectors. The alpha particles it emits ionise the air molecules, which then can conduct an electric current between two terminals. If smoke is present it will cling to the air molecules inside the smoke alarm and slows them down so the current decreases and a switch activates the alarm.
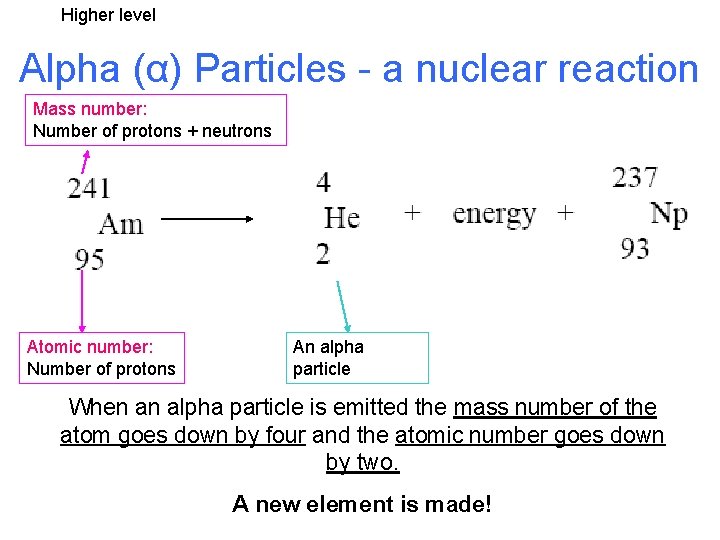
Higher level Alpha (α) Particles - a nuclear reaction Mass number: Number of protons + neutrons Atomic number: Number of protons An alpha particle When an alpha particle is emitted the mass number of the atom goes down by four and the atomic number goes down by two. A new element is made!
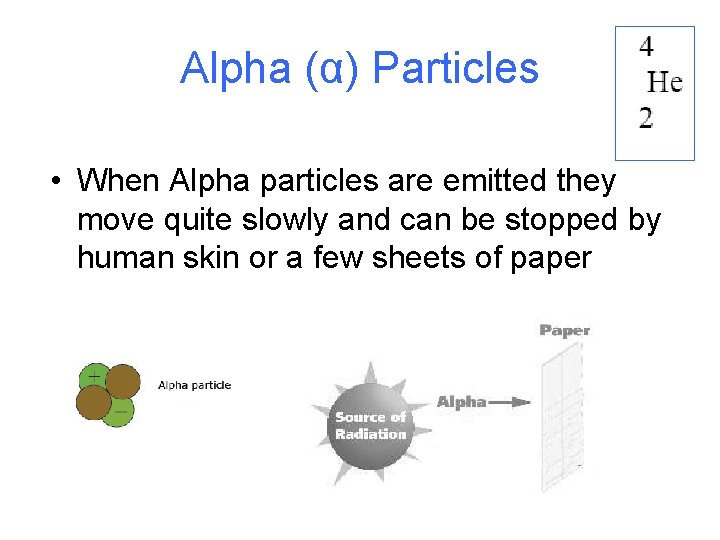
Alpha (α) Particles • When Alpha particles are emitted they move quite slowly and can be stopped by human skin or a few sheets of paper
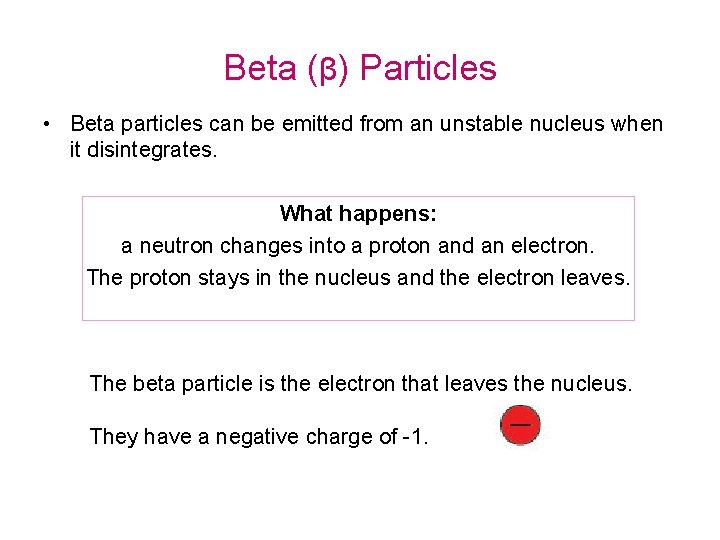
Beta (β) Particles • Beta particles can be emitted from an unstable nucleus when it disintegrates. What happens: a neutron changes into a proton and an electron. The proton stays in the nucleus and the electron leaves. The beta particle is the electron that leaves the nucleus. They have a negative charge of -1.
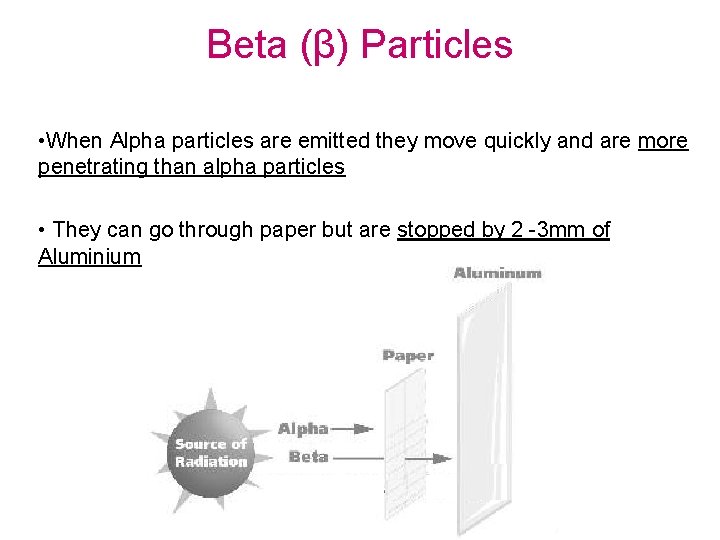
Beta (β) Particles • When Alpha particles are emitted they move quickly and are more penetrating than alpha particles • They can go through paper but are stopped by 2 -3 mm of Aluminium
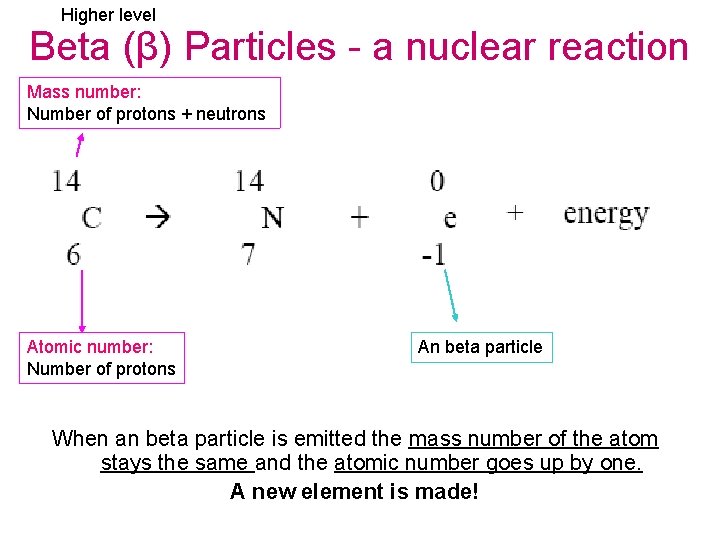
Higher level Beta (β) Particles - a nuclear reaction Mass number: Number of protons + neutrons Atomic number: Number of protons An beta particle When an beta particle is emitted the mass number of the atom stays the same and the atomic number goes up by one. A new element is made!
Nuclear reactions • We have already seen two examples of nuclear reactions involving alpha particles and beta particles • Nuclear reactions cause changes in the nucleus – they involve protons and neutrons • They cause elements to change into other elements Nuclear reactions are very different to chemical reactions where one element cannot be changed into any other
Carbon-14 is a radioactive isotope that emits beta particles when it decays. Carbon dating is a method used for estimating the age of materials that contain carbon – like paintings, fabric and wood. When an organism is alive it contains carbon-12 and carbon-14 in the proportions it is present in the air After the death of an organism the unstable carbon-14 decays but the stable carbon -12 stays the same. By measuring the ratio of carbon-12 levels to carbon-14 levels in an object the age can be estimated.
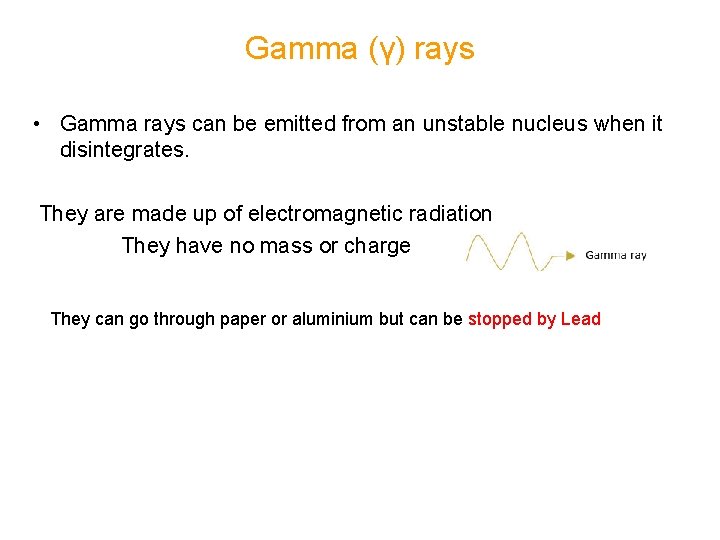
Gamma (γ) rays • Gamma rays can be emitted from an unstable nucleus when it disintegrates. They are made up of electromagnetic radiation They have no mass or charge They can go through paper or aluminium but can be stopped by Lead
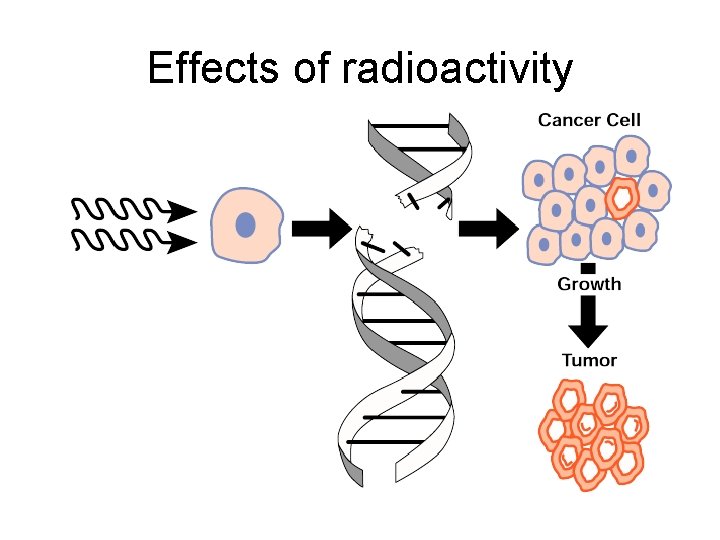
Effects of radioactivity
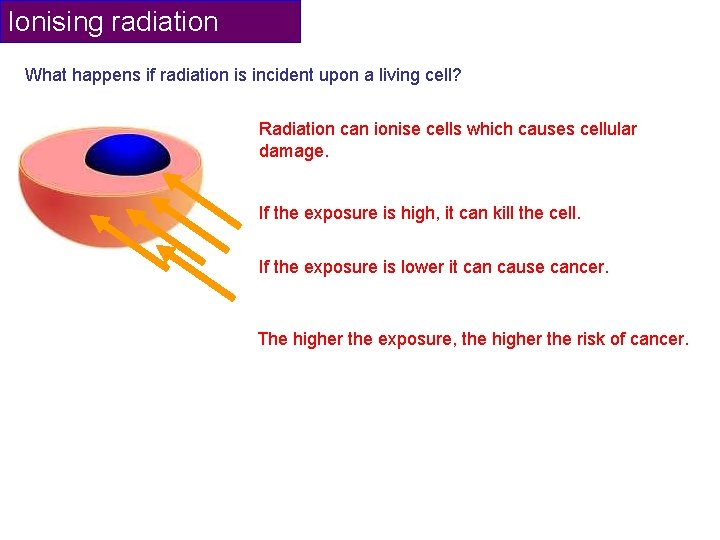
Ionising radiation What happens if radiation is incident upon a living cell? Radiation can ionise cells which causes cellular damage. If the exposure is high, it can kill the cell. If the exposure is lower it can cause cancer. The higher the exposure, the higher the risk of cancer.

Radiotherapy A carefully controlled beam of gamma rays can be used to kill cancer cells. It must be directed carefully to minimise the damage to normal cells. However, some damage is unavoidable and this can make the patient ill. It is therefore a balancing act - getting the dose high enough to kill the cancerous cells, but as low as possible to minimise the harm to the patient.
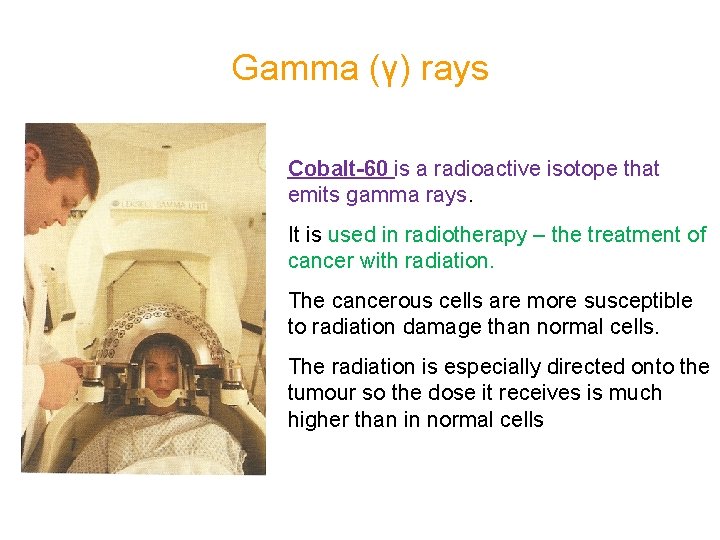
Gamma (γ) rays Cobalt-60 is a radioactive isotope that emits gamma rays. It is used in radiotherapy – the treatment of cancer with radiation. The cancerous cells are more susceptible to radiation damage than normal cells. The radiation is especially directed onto the tumour so the dose it receives is much higher than in normal cells
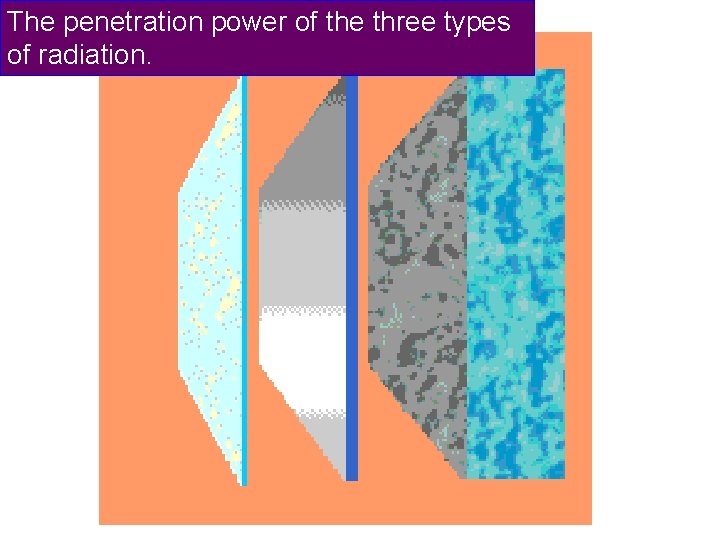
The penetration power of the three types of radiation.
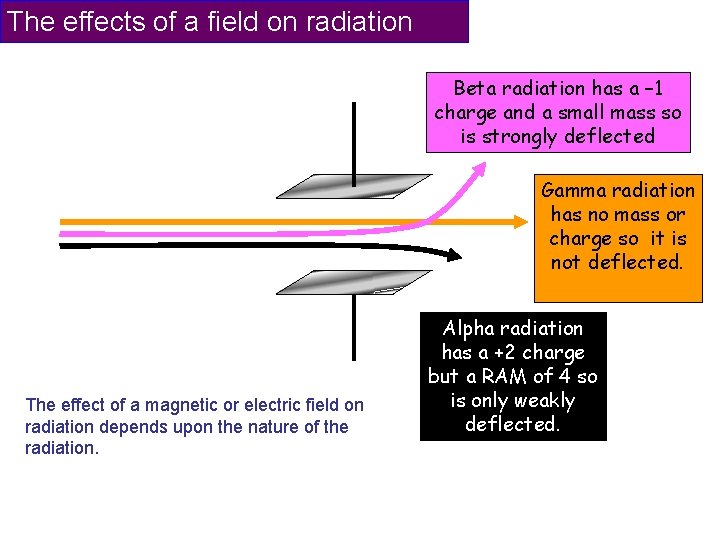
The effects of a field on radiation Beta radiation has a – 1 charge and a small mass so is strongly deflected Gamma radiation has no mass or charge so it is not deflected. The effect of a magnetic or electric field on radiation depends upon the nature of the radiation. Alpha radiation has a +2 charge but a RAM of 4 so is only weakly deflected.

Radioisotopes • Most elements have isotopes, sometimes the isotopes are radioactive. • Unstable radioactive isotopes are called radioisotopes • Examples – • Carbon – 14 • Americium -241 • Caobalt - 60

Half life • It is impossible to tell when a radioactive atom will decay • Def Half life is the time taken for half of the radioactive atoms in a sample to decay. • Half life's vary : radium-214 has a half life of 20 minutes while raduim-226 has a half life of 1, 620 years!
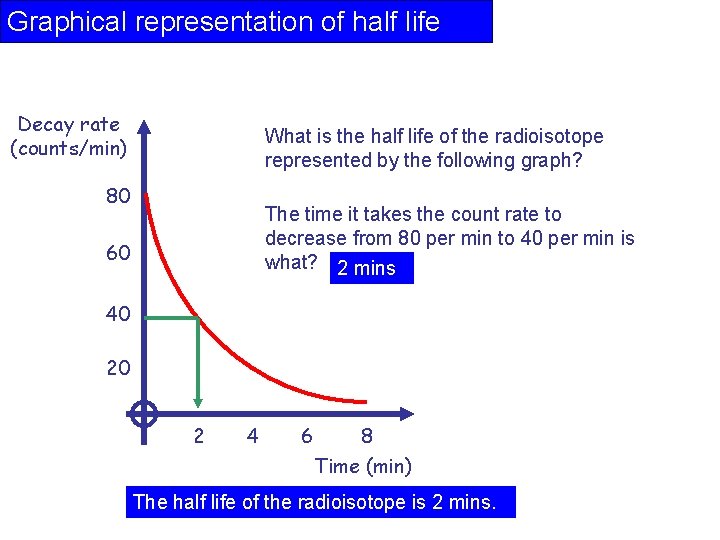
Graphical representation of half life Decay rate (counts/min) What is the half life of the radioisotope represented by the following graph? 80 The time it takes the count rate to decrease from 80 per min to 40 per min is what? 2 mins 60 40 20 2 4 6 8 Time (min) The half life of the radioisotope is 2 mins.

Some of the radioactive waste from nuclear power plants has a half life of millions of years…. Is safe disposal of such material achievable?

Radioactive waste The current solutions are: 1. Store it at the nuclear power station until is filled up. 2. Dump it far out at sea. 3. Store it deep underground in nonpermeable rock.
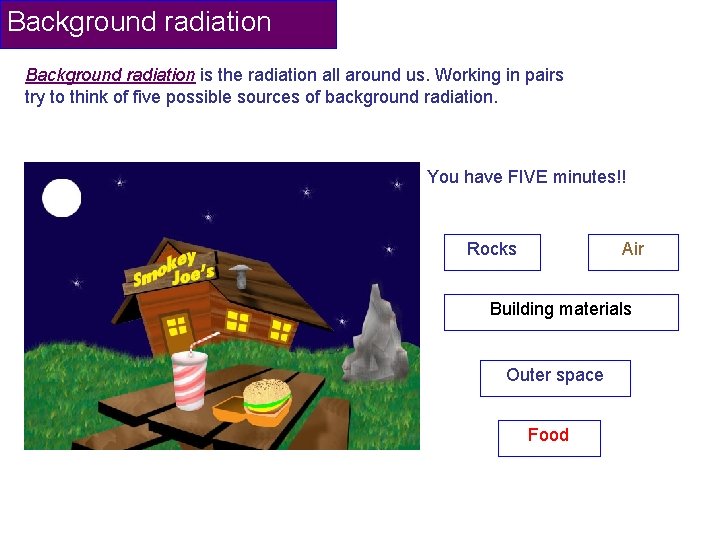
Background radiation is the radiation all around us. Working in pairs try to think of five possible sources of background radiation. You have FIVE minutes!! Rocks Air Building materials Outer space Food
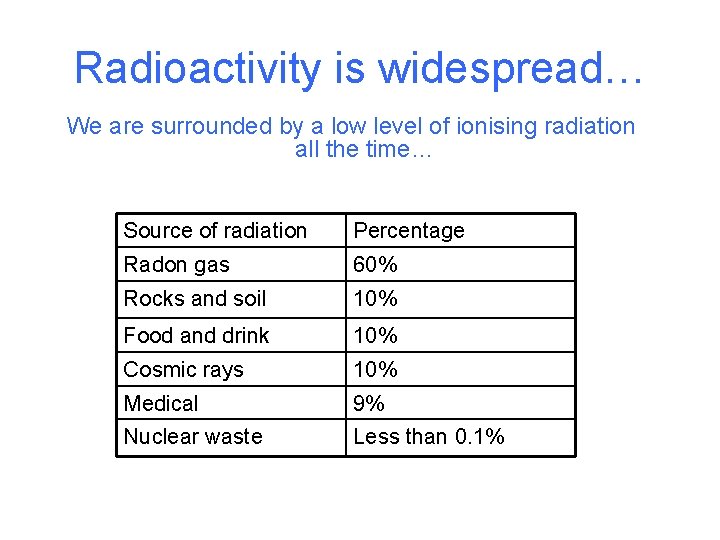
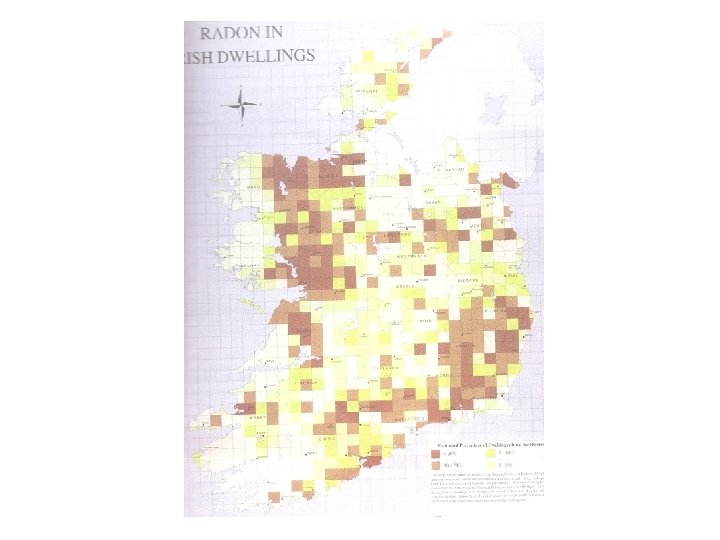
Radioactivity is widespread… We are surrounded by a low level of ionising radiation all the time… Source of radiation Percentage Radon gas 60% Rocks and soil 10% Food and drink 10% Cosmic rays 10% Medical 9% Nuclear waste Less than 0. 1%
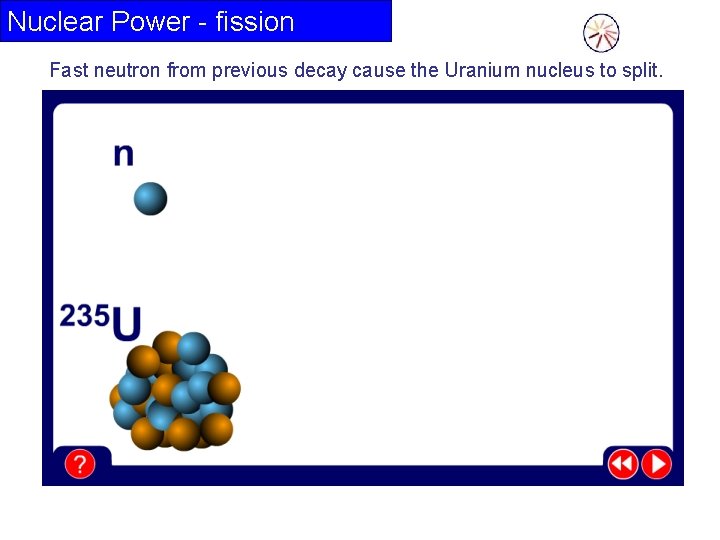
Nuclear Power - fission Fast neutron from previous decay cause the Uranium nucleus to split.
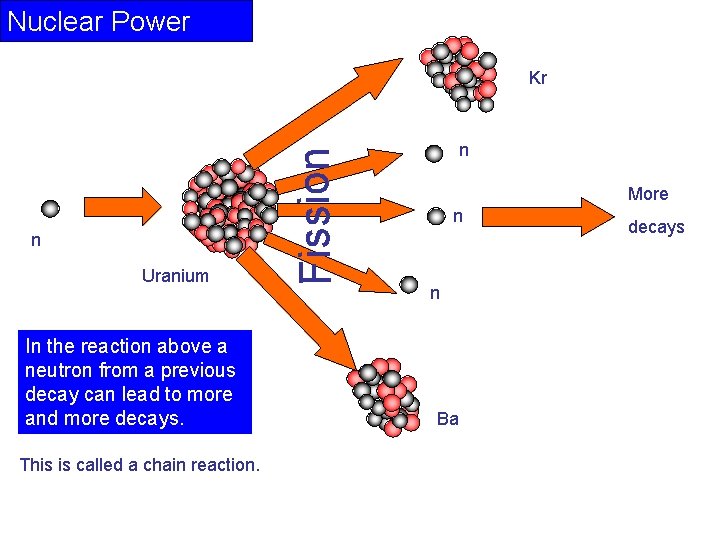
Nuclear Power n Uranium In the reaction above a neutron from a previous decay can lead to more and more decays. This is called a chain reaction. Fission Kr n More n n Ba decays
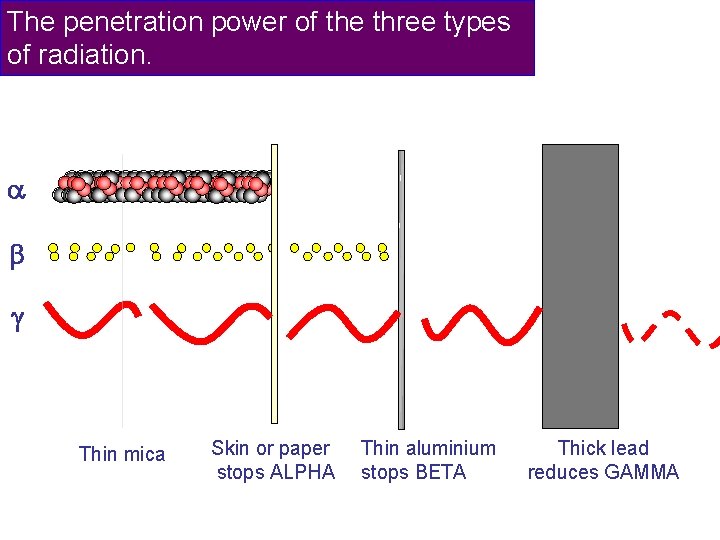
The penetration power of the three types of radiation. Thin mica Skin or paper stops ALPHA Thin aluminium stops BETA Thick lead reduces GAMMA
- Slides: 42