Radioactivity Ch 10 I Radioactivity A is the






























- Slides: 34

Radioactivity Ch. 10

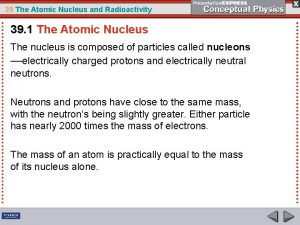
I. Radioactivity A. is the process in which an unstable atomic nucleus emits charged particles and energy B. Any atom containing an unstable nucleus is called a radioactive isotope or radioisotope

Examples: 39 II. Nuclides and Isotopes 40 K & K 19 19 14 C & 12 C 6 6 A. Superscript is the mass number B. Subscript is the atomic number

C. Isotopes 1. have the same number of protons, different number of neutrons 2. Another way to show an isotope is to have the mass number follow the name of the element (Carbon-14 or C-14)

III. Types of Nuclear Radiation A. Alpha B. Beta C. Gamma

IV. Alpha Decay A. Alpha particle—a positively charged particle made up of two protons and two neutrons – 1. the least penetrating – 2. can be stopped by a sheet of paper

IV. Alpha Decay continued. . . B. An alpha particle looks like a helium atom (42 He) C. During alpha decay, mass reduces by 4 , the atomic # reduces by 2 – Examples: 238 U 92 209 Po 84


V. Beta Decay A. A beta particle is an electron emitted by an unstable nucleus 1. can be stopped by a thin sheet of metal such as aluminum

V. Beta Decay continued 0 B. A beta particle is written -1 e C. During beta decay, mass remains the same & the atomic # increases by one – Examples: 214 218 82 84 Pb Po


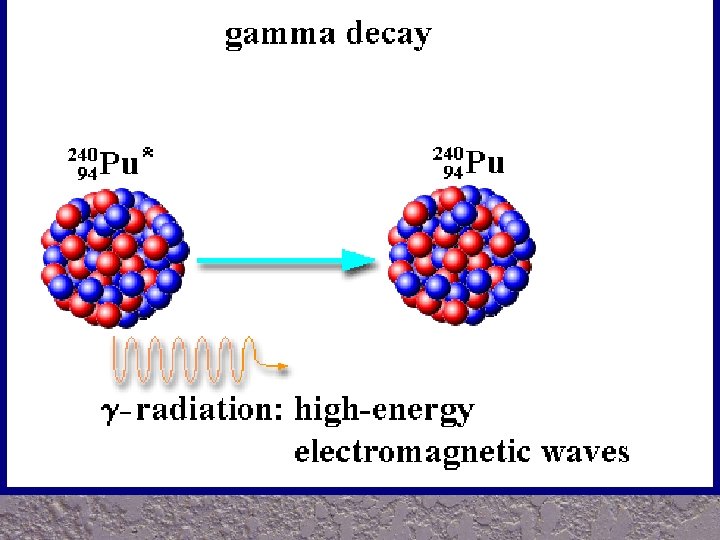
VI. Gamma decay A. A gamma ray is a penetrating ray of energy emitted by an unstable nucleus – Gamma rays are energy waves that travel through space at the speed of light

VI. Gamma decay continued B. During gamma decay, atomic # and mass remain the same, but the energy of nucleus decreases C. Gamma rays can be stopped by: 1. several centimeters of lead 2. several meters of concrete


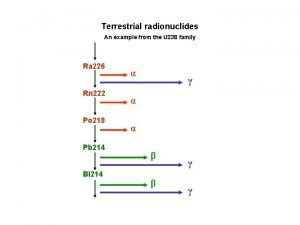
VII. Sources of Nuclear Radiation A. Background radiation is nuclear radiation that occurs naturally in the environment 1. Radioisotopes in the air, water, rocks, plants, and animals all contribute to background radiation

VII. Sources of Nuclear Radiation 2. Cosmic rays (streams of charged particles) from outer space that collide with the Earth’s atmosphere also contribute 3. Background radiation levels are low enough to be safe 4. When nuclear radiation exceeds background levels, cells in your body can mutate.

Gamma Radiation looks like this…

5. Detecting Radiation • Devices used to detect radiation include Geiger counters and film badges

VIII. RATES OF NUCLEAR DECAY A. A half-life is the time required for one half of a sample of a radioisotope to decay 1. Unlike chemical reactions, nuclear decay rates are constant regardless of temperature, pressure or surface area a. How much of the original sample will remain after 5 half-lives?

RATES OF NUCLEAR DECAY B. Transmutation is the conversion of atoms of one element to atoms of another C. Transuranium elements are elements with atomic numbers higher than 92 D. A quark is a subatomic particle theorized to be among the basic units of matter

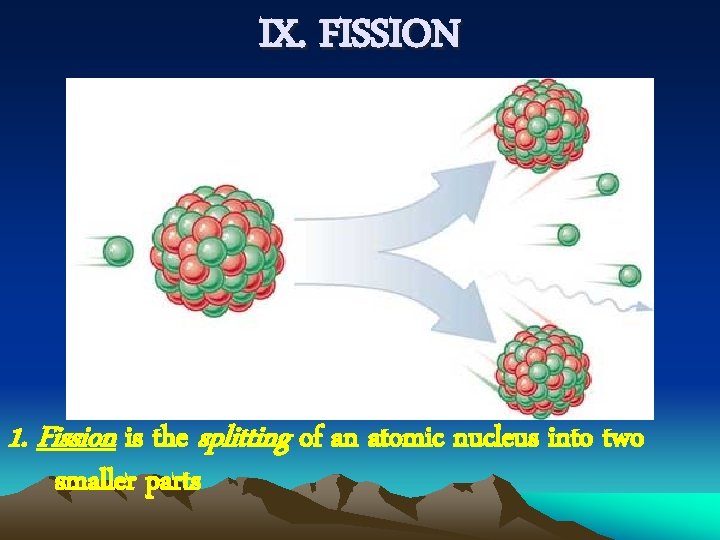
IX. FISSION 1. Fission is the splitting of an atomic nucleus into two smaller parts

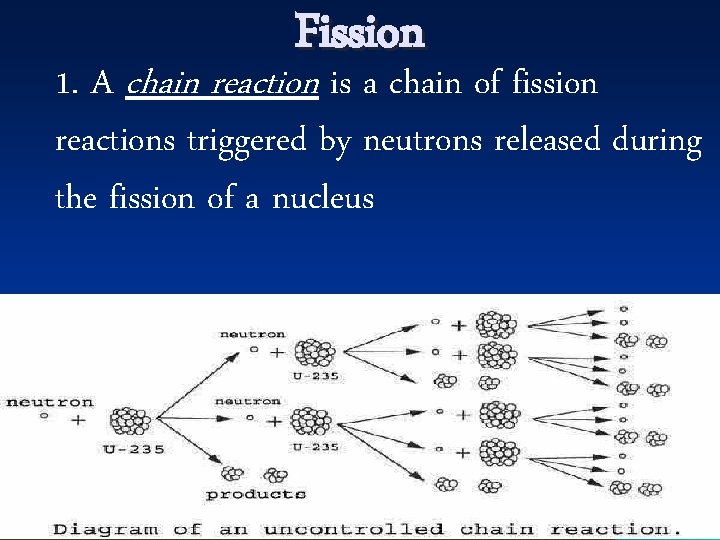
Fission 1. A chain reaction is a chain of fission reactions triggered by neutrons released during the fission of a nucleus

2. A tremendous amount of energy is produced during a fission reaction 3. About 20% of the electricity in the US comes from fission reactions Fission

Fission 4. An advantage to using fission reactions is the lack of air pollution. 5. Disadvantages include the risk of exposure and radioactive waste. Harris plant, near Raleigh Lake Harris NIMBY



Radioactive Waste Can Cause…

X. Fusion A. Fusion is a process in which the nuclei of two atoms combine to form a larger nucleus 1. Fusion reactions can release huge amounts of energy 2. Fusion reactions occur in the sun and stars (plasma)

Fusion 3. We do not use fusion reactions for energy b/c of the extremely high temperatures needed to start the reaction & because the plasma would need to be contained.

• Page 13: Beta Decay – Define Beta Decay (nuclear decay that releases electrons from an unstable nucleus) – Draw the symbol for beta decay – Draw what it can be stopped by. – Balance the following beta decay equations: – 238 U 210 At 226 Ra 92 85 88

Page 14: Fission vs. Fusion –For both FISSION and FUSION, d –Define and draw a picture and write where each occurs

Physi. Facts 3. 4 2/28 • 12. Define isotope. • 13. What element does an alpha particle look like? • Use your solubility curve (IAN page 11) to answer the following questions: • 14. Which two substances have the same solubility at 30 degrees Celsius? • 15. If 30 g of KCl is dissolved in 100 g of water at 30 degrees Celsius, what type of solution is formed?

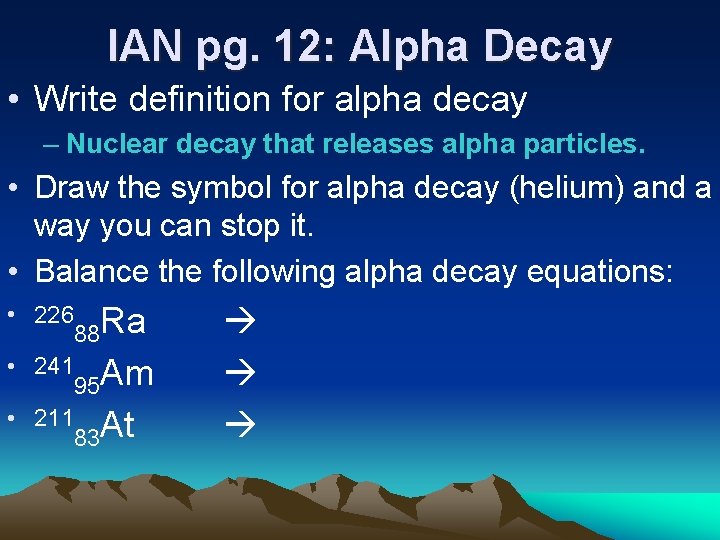
IAN pg. 12: Alpha Decay • Write definition for alpha decay – Nuclear decay that releases alpha particles. • Draw the symbol for alpha decay (helium) and a way you can stop it. • Balance the following alpha decay equations: • 226 88 Ra • 241 95 Am • 211 83 At

Unit 1 Task Cards • You and a partner will receive one task card. • Write the number and answer the question in a complete sentence OR write the question • When finished with that card, swap with a group at your table, or at the next table over. Please do not wander around the room. • You have 30 minutes to complete a minimum of TEN – anything over 10 will be bonus points towards your test. Get as many done as you can!
 The great unconformity
The great unconformity Who discovered radioactivity
Who discovered radioactivity Natural radioactivity
Natural radioactivity Units of radioactivity
Units of radioactivity Radioactivity as spontaneous disintegration
Radioactivity as spontaneous disintegration Natural and artificial radioactivity
Natural and artificial radioactivity Defination of radioactivity
Defination of radioactivity Datación radiométrica
Datación radiométrica Who discovered uranium
Who discovered uranium Radioactive decay equation
Radioactive decay equation Radioactivity
Radioactivity Natural and artificial radioactivity
Natural and artificial radioactivity Beta decay of carbon
Beta decay of carbon Radioactivity phenomenon
Radioactivity phenomenon Natural radioactivity
Natural radioactivity Natural and artificial radioactivity
Natural and artificial radioactivity Defination of radioactivity
Defination of radioactivity Radioactivity
Radioactivity Who discovered radioactivity
Who discovered radioactivity Environmental radioactivity
Environmental radioactivity Key terms radioactivity and nuclear reactions
Key terms radioactivity and nuclear reactions Natural vs artificial radioactivity
Natural vs artificial radioactivity Defination of radioactivity
Defination of radioactivity Một số thể thơ truyền thống
Một số thể thơ truyền thống Lời thề hippocrates
Lời thề hippocrates Hổ đẻ mỗi lứa mấy con
Hổ đẻ mỗi lứa mấy con Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ đại từ thay thế
đại từ thay thế Ng-html
Ng-html Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Glasgow thang điểm
Glasgow thang điểm Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các loại đột biến cấu trúc nhiễm sắc thể
Các loại đột biến cấu trúc nhiễm sắc thể Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể