Radioactivity and Nuclear Decay Test on Friday March






















- Slides: 32

Radioactivity and Nuclear Decay Test on Friday March 1

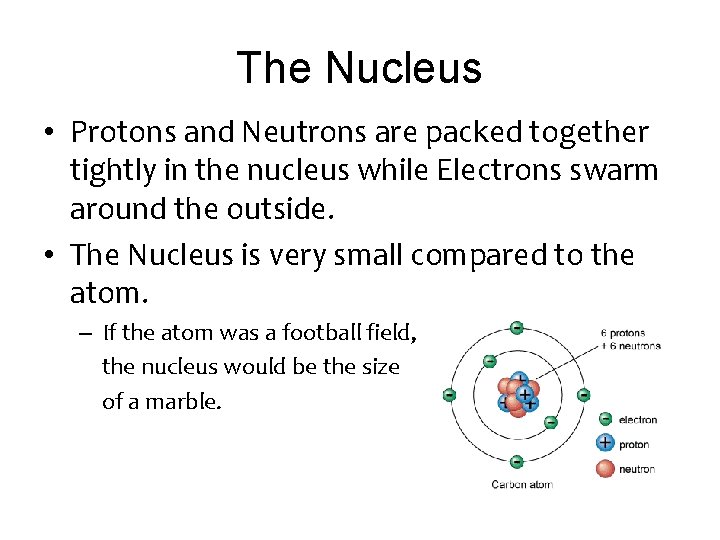
The Nucleus • Protons and Neutrons are packed together tightly in the nucleus while Electrons swarm around the outside. • The Nucleus is very small compared to the atom. – If the atom was a football field, the nucleus would be the size of a marble.

The Nucleus • The Nucleus (Protons and Neutrons) is held together by the Strong Nuclear Force (AKA Strong Force) • The strong force is an attractive force that is 100 times stronger than the electric force • The strong force only acts over short distances. • Since the nuclear force is so strong, there is lots of energy stored in the nucleus

Nuclear Size • Bigger nuclei are held together less strongly than smaller nuclei. • Therefore, bigger nuclei aren’t as stable as smaller nuclei.

Radioactivity • Most of the time, the strong force is able to hold the nucleus together. • If the nucleus is too big, the strong force can’t hold it together and the atom is unstable. • Unstable atoms have a nucleus that decays and gives off matter and energy. • The process of nuclear decay is called radioactivity.

Radioactive Elements • All atoms that have an atomic number bigger than 83 are radioactive • In other words, if a nucleus has more than 83 protons in it, the strong force cannot hold the nucleus together and it will decay. • Elements that have more than 92 protons don’t exist naturally on earth. They are called synthetic elements and decay soon after being produced in a lab.

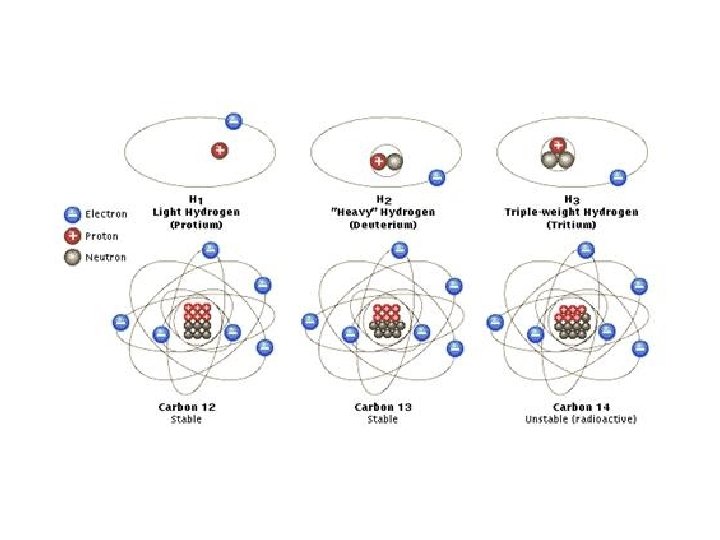
Isotope • An atom is an isotope if the number of protons and the number of neutrons are different. • Some elements have naturally occurring isotopes. (Example Carbon 12, Carbon 13, and Carbon 14)


Nuclear Ratios • To determine the ratio of neutrons to protons: • Divide the number of neutrons by the number of protons. • For a normal atom (non-isotope), the ratio will always be 1: 1

Radioactive Isotopes • Isotopes are stable when the ratio of neutrons to protons is 3: 2 • If the ratio is much different from this, the isotope will be radioactive. • In other words, nuclei with too few or too many neutrons compared to the protons is radioactive.

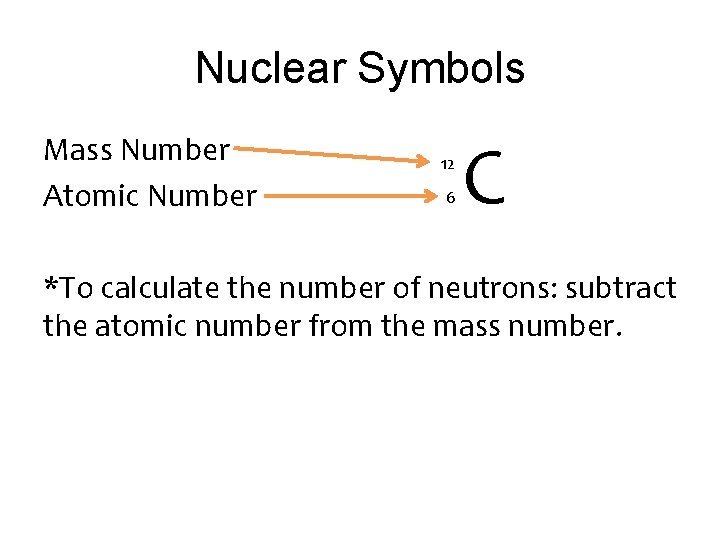
Nuclear Numbers • A nucleus can be described by the number of protons and neutrons that it contains. • The number of protons is called the atomic number • The number of protons plus neutrons is called the mass number.

Nuclear Symbols Mass Number Atomic Number 12 6 C *To calculate the number of neutrons: subtract the atomic number from the mass number.

Discovery • In 1896 Henri Becquerel left uranium on a film, later he saw an outline of the uranium. • Two years later, Marie and Pierre Curie discovered two new elements (Polonium and Radium) that were radioactive. • They developed a process to extract the radioactive elements from rocks.

Nuclear Radiation • When a nucleus decays, particles and energy are emitted from it. These particles are called nuclear radiation. – Nuclear radiation consists of alpha, beta, and gamma particles.

Alpha Particles • Made of two protons and two neutrons. • Has a charge of +2 and a mass of 4. • Leave charged ions behind when they travel through matter • Because alpha particles have the biggest mass and the biggest charge of all radiation, they lose energy very quickly. • They are the least penetrating of the radiation particles and can be stopped by a single sheet of paper.

Beta Particles • When a neutron decays into a proton and emits one electron • Has a charge of -1 and a mass of 0. 0005 • Caused by the weak force • Much faster than alpha particles and more penetrating. • They can be stopped by a sheet of aluminum foil.

Gamma Rays • • • Electromagnetic Waves No charge and no mass Most penetrating form of nuclear radiation Travel at the speed of light Least damaging radiation Can be stopped by lead or concrete

Transmutation • The process of changing from one element to another through nuclear decay. • In Alpha radiation, an atom loses two protons. Its atomic number decreases by two. • In Beta radiation, at atom gains a proton. Its atomic number increases by one.

Half Life • A measure of the time it takes for half of a radioactive isotope to decay. • The nucleus left after the isotope decays is called the daughter nucleus.

Radioactive Dating • If we know the half life of an element and what percent of the sample remains unchanged (still radioactive) we can tell how long the sample has been there. • Carbon-14 is used for living or once living things • Uranium is used for rocks and non-living things.

Radiation Detectors • Radiation can be detected with – Cloud Chambers – Bubble Chambers – Electroscopes • These devices only detect if radiation is present, they don’t measure the amount. • They detect ions formed when radiation passes through matter.

Cloud Chambers • A sample is placed into a chamber filled with vapor. If the particle is radioactive, the radiation given off travels through the vapor and leaves a visible ion trail.

Bubble Chamber • A chamber is filled with superheated liquid. As the radioactive sample decays, the ion trail causes the liquid to boil.

Electroscope • The leaves of an electroscope spread apart in the presence of radiation. • They are used to detect charged particles in the air.

Measuring Radiation • The amount of radiation present can be measured with a Geiger Counter. • A GC produces an electric current in the presence of radiation. The more radiation present, the bigger the current.

Background Radiation • Low level radiation emitted by naturally occurring radioactive isotopes in the earth’s rocks, soil and atmosphere. • The largest source of radiation is Radon Gas (from the decay of Uranium)

Nuclear Reactions • There are two types of nuclear reactions: – Fission – Fusion

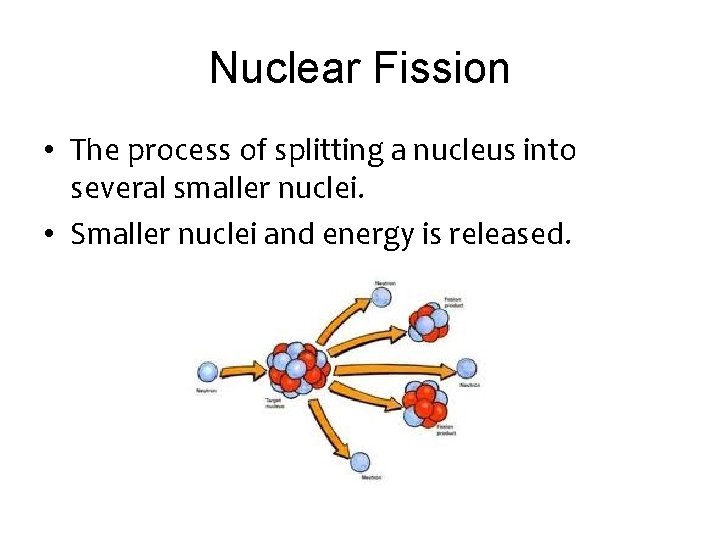
Nuclear Fission • The process of splitting a nucleus into several smaller nuclei. • Smaller nuclei and energy is released.

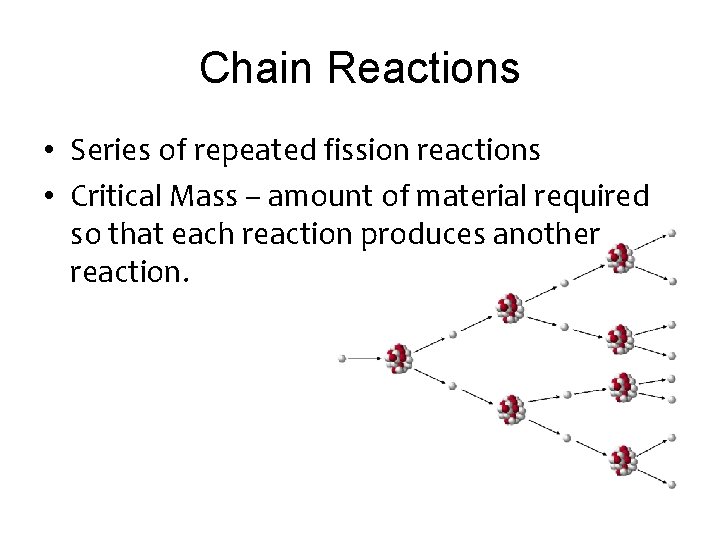
Chain Reactions • Series of repeated fission reactions • Critical Mass – amount of material required so that each reaction produces another reaction.

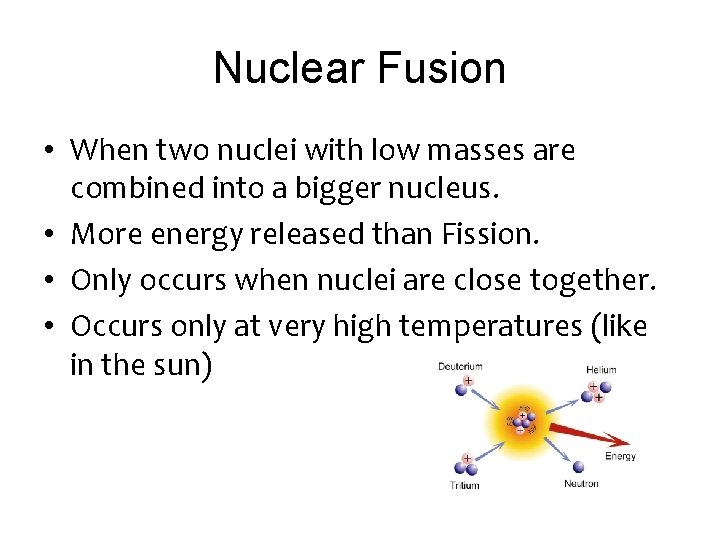
Nuclear Fusion • When two nuclei with low masses are combined into a bigger nucleus. • More energy released than Fission. • Only occurs when nuclei are close together. • Occurs only at very high temperatures (like in the sun)

Nuclear Fusion in the Sun • As the sun ages, the hydrogen nuclei are converted to helium. • Eventually there will be no hydrogen left and the sun will burn out.

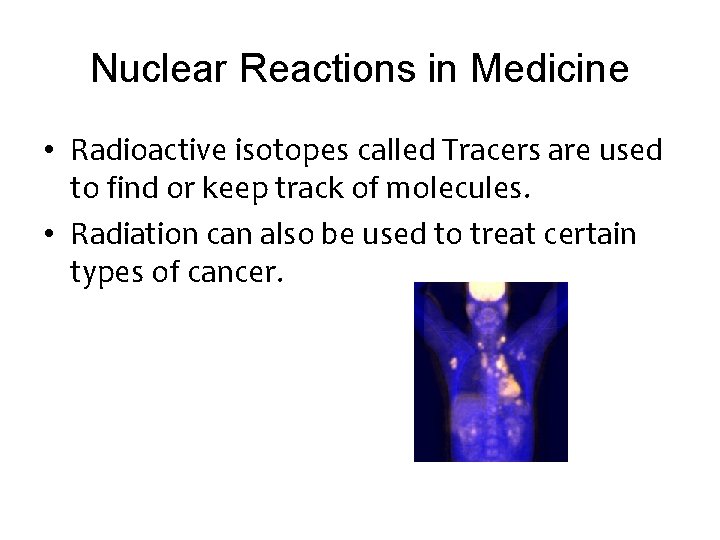
Nuclear Reactions in Medicine • Radioactive isotopes called Tracers are used to find or keep track of molecules. • Radiation can also be used to treat certain types of cancer.