Radioactive materials An atom proton electron neutron 0







- Slides: 16

Radioactive materials

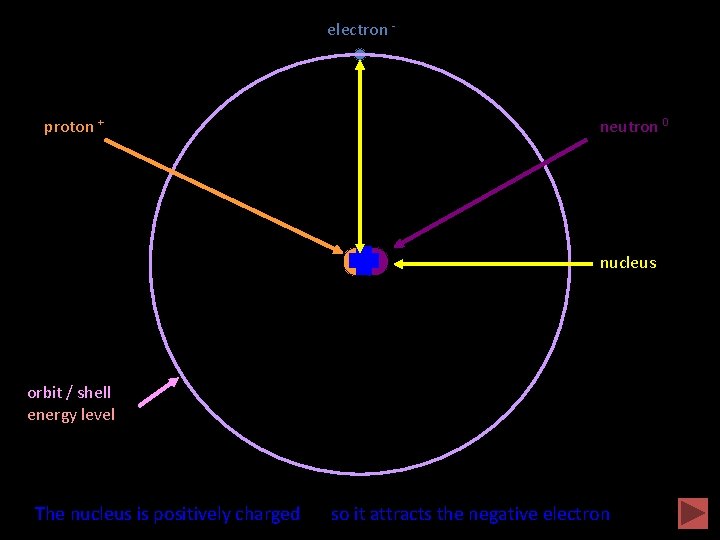
An atom proton + electron - neutron 0 nucleus orbit / shell energy level Animation completed The nucleus is positively charged so it attracts the negative electron

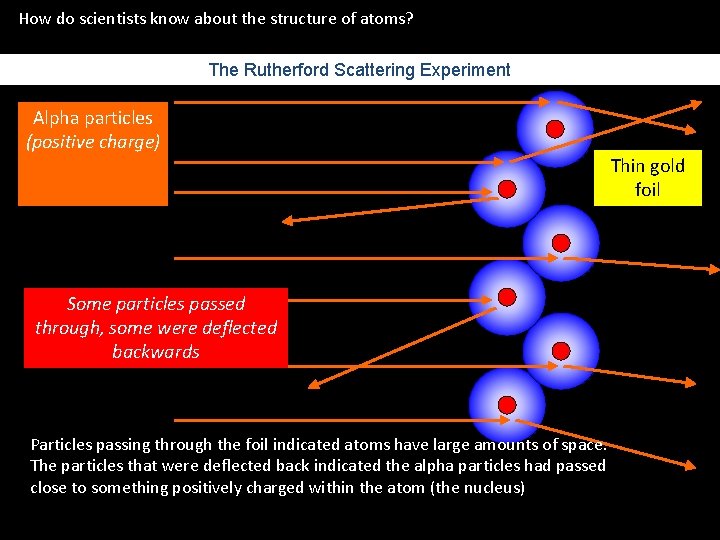
How do scientists know about the structure of atoms? The Rutherford Scattering Experiment Alpha particles (positive charge) Some particles passed through, some were deflected backwards Particles passing through the foil indicated atoms have large amounts of space. The particles that were deflected back indicated the alpha particles had passed close to something positively charged within the atom (the nucleus) Thin gold foil

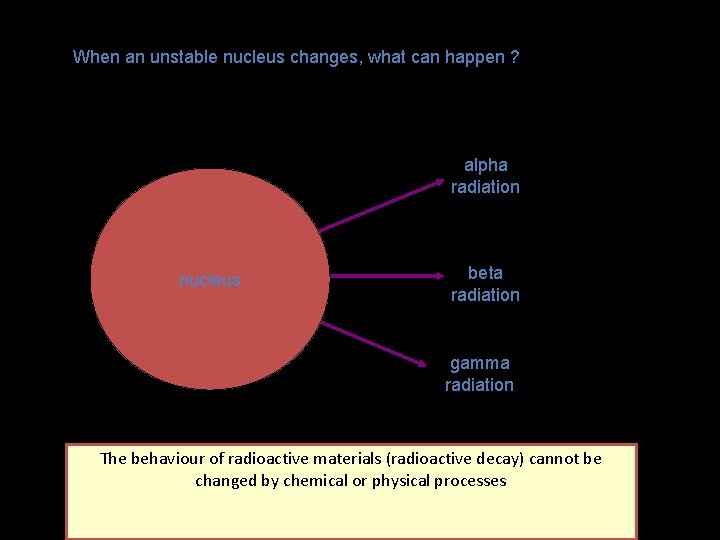
When an unstable nucleus changes, what can happen ? alpha radiation nucleus beta radiation gamma radiation Radioactive isotopes release radiation and the nucleus changes The behaviour of radioactive materials (radioactive decay) cannot be changed by chemical or physical processes

Isotopes An isotope is an atom with a different number of neutrons: A “radioisotope” is simply an isotope that is radioactive – e. g. carbon 14, which is used in carbon dating. Radioactive changes – some nuclei that are unstable can become stable by emitting an alpha or beta particle 1) Alpha ( ) – an atom decays into a new atom and emits an alpha particle (2 protons and 2 neutrons – the nucleus of a helium atom) 2) Beta ( ) – an atom decays into a new atom by changing a neutron into a proton and electron. The fast moving, high energy electron is called a beta particle. 3) Gamma – after or decay surplus energy is sometimes emitted. This is called gamma radiation and has a very high frequency with short wavelength. The atom is not changed.

Alpha decay Example Radium-226 undergoing alpha decay forms Radon-222, an alpha particle and releases energy. Beta decay Example Polonium-218 undergoing beta decay forms Astatine-218, an electron and releases energy.

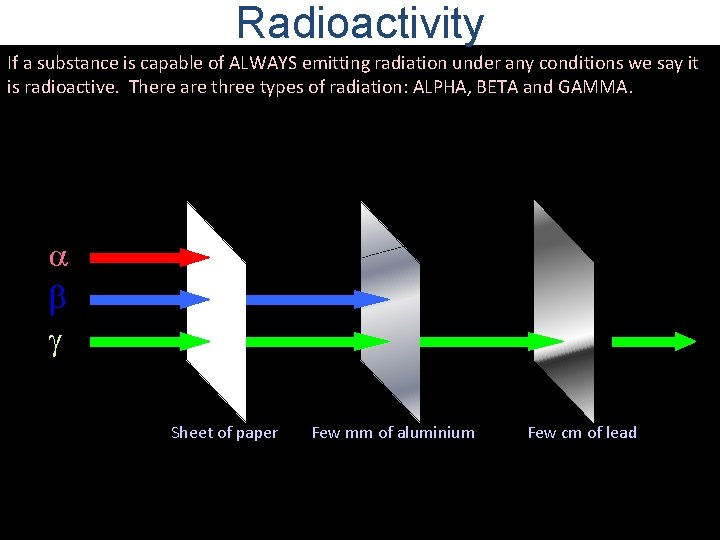
Radioactivity If a substance is capable of ALWAYS emitting radiation under any conditions we say it is radioactive. There are three types of radiation: ALPHA, BETA and GAMMA. Sheet of paper Few mm of aluminium Few cm of lead

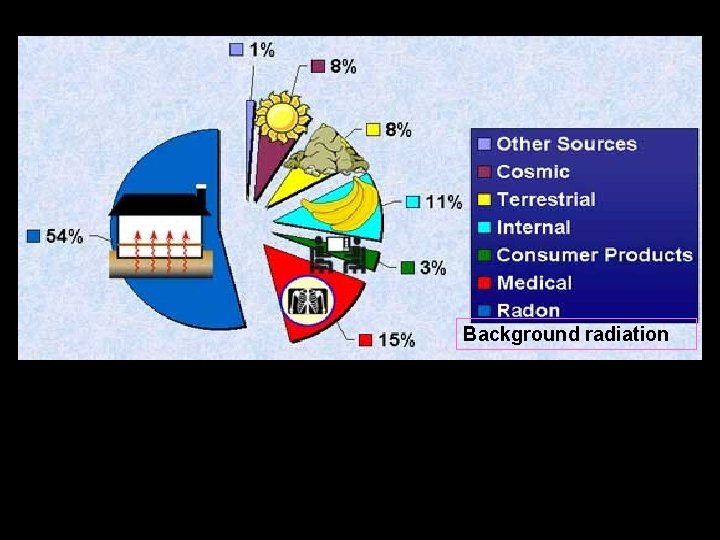
Sources of background radiation Background radiation Radiation dose measures the possible harm the radiation could do to the body. It is measures in millisieverts (m. Sv). The potential harm done depends on • the amount of radiation • the type of radiation

Exposure to a radiation source outside your body is called irradiation If a radiation source enters your body, or gets on skin or clothes, it is called contamination Alpha particles are the most ionising so they are the most dangerous inside your body Employers must ensure that radiation workers receive a Radiation dose “as low as reasonably achievable”. Precautions taken are • use protective clothing and screens • . wear gloves and aprons • wear special devices to monitor their dose

Uses of gamma radiation treating cancer it can kill cancer cells sterilising equipment it can penetrate the outer casing and kill microbes sterilising food it can kill microbes without harming the food

Radon gas is harmful because it is radioactive. It produces ionising radiation that can damage cells. Medical imaging and treatment Radioactive materials cane be used to diagnose and cure many health problems. Radiotherapy is used to kill cancer cells

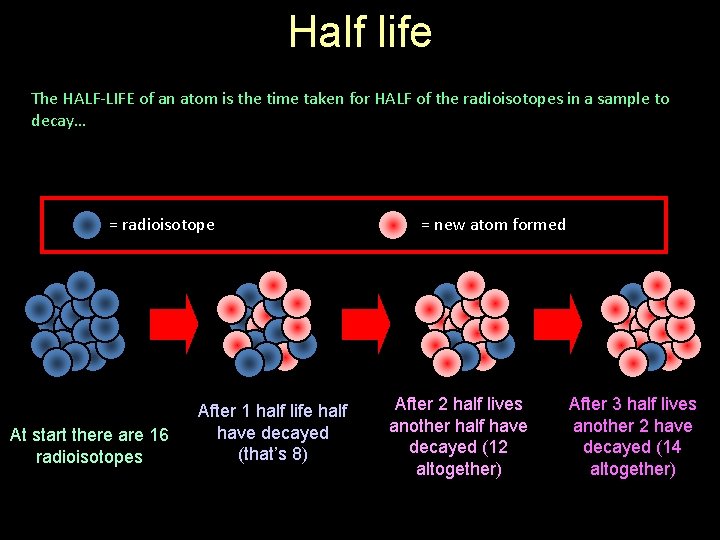
Half life The HALF-LIFE of an atom is the time taken for HALF of the radioisotopes in a sample to decay… = radioisotope At start there are 16 radioisotopes After 1 half life half have decayed (that’s 8) = new atom formed After 2 half lives another half have decayed (12 altogether) After 3 half lives another 2 have decayed (14 altogether)

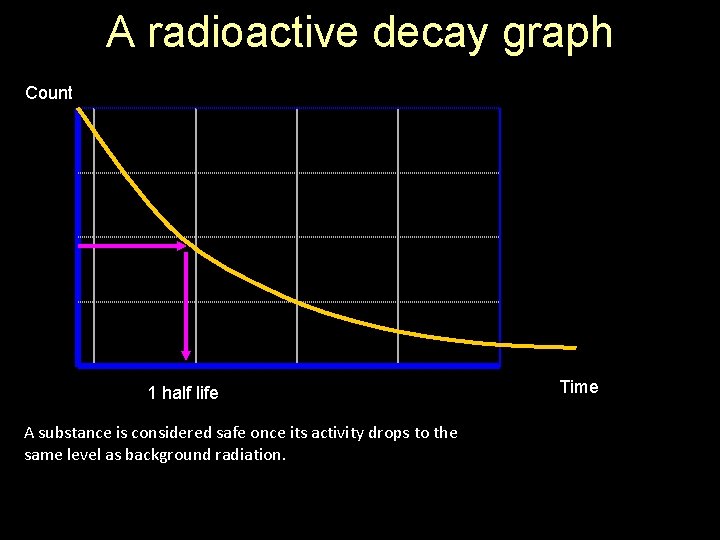
A radioactive decay graph Count 1 half life A substance is considered safe once its activity drops to the same level as background radiation. Time

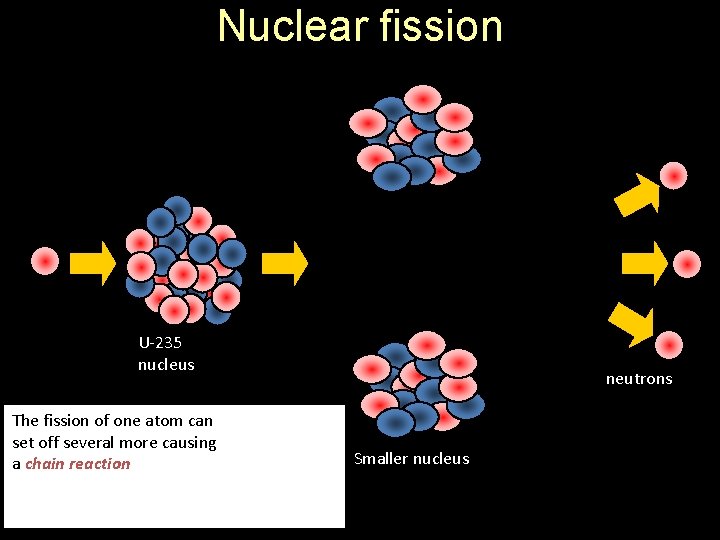
Nuclear fission The energy released can be calculated from Einstein’s equation : E = mc² ENERGY neutron U-235 nucleus The fission of one atom can set off several more causing a chain reaction neutrons Smaller nucleus

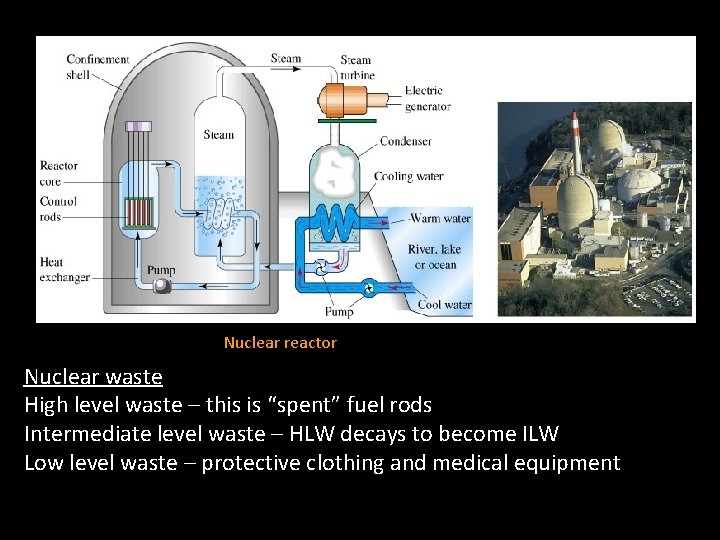
Nuclear reactor Nuclear waste High level waste – this is “spent” fuel rods Intermediate level waste – HLW decays to become ILW Low level waste – protective clothing and medical equipment

Nuclear fusion – the nuclei of two hydrogen atoms join together and energy is released. Protons and neutrons in a nucleus are held together by a strong nuclear force, which acts against the electrical repulsive force between protons