Radioactive Decay Review of Atomic Structure Electron negatively






- Slides: 20

Radioactive Decay

Review of Atomic Structure ª Electron: negatively charged fundamental particle ª Proton: positively charged fundamental particle ª Neutron: uncharged fundamental particle ª Nucleus: small, central unit in the atom that contains neutrons and protons

Atomic Mass Nucleons are any particles found in the nucleus in an atom, which include… ª Protons- 1 atomic mass unit (amu); + charge ª Neutrons- 1 atomic mass unit (amu); 0 charge w NOTE: Neutrons are essentially a Proton/Electron “bundle” Electrons have essentially no mass; - charge

Stable Atoms ª A atom’s nucleus is stable if the strong force is sufficient to hold the nucleons together ª Instability can be caused by in imbalance of protons & neutrons or the nucleus being too large ª Instability is random

Becoming Stable An unstable nucleus will attempt to reach stability by some combination of means: ª Ejecting neutrons and protons (alpha particles) ª Converting one to the other with the ejection of a high energy particle (beta particles or positrons) ª The release of additional energy by photon (gamma ray) emission. ALL of these processes release energy and are considered as nuclear radiation

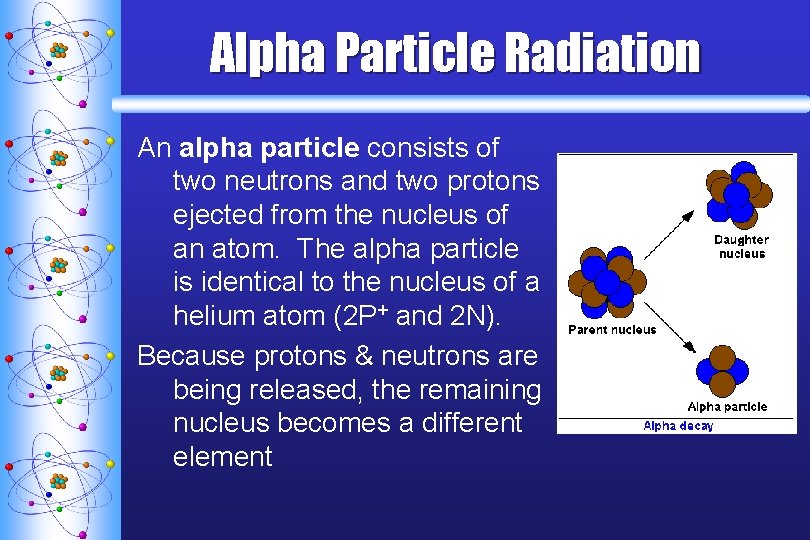
Alpha Particle Radiation An alpha particle consists of two neutrons and two protons ejected from the nucleus of an atom. The alpha particle is identical to the nucleus of a helium atom (2 P+ and 2 N). Because protons & neutrons are being released, the remaining nucleus becomes a different element

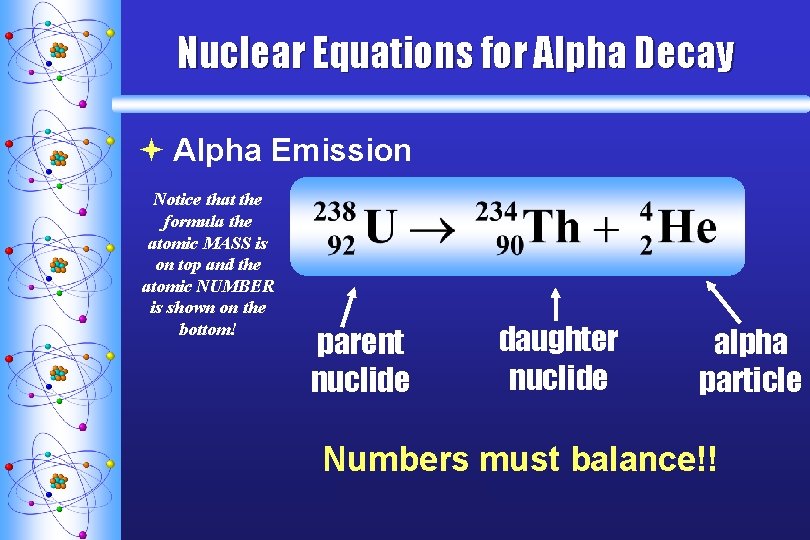
Nuclear Equations for Alpha Decay ª Alpha Emission Notice that the formula the atomic MASS is on top and the atomic NUMBER is shown on the bottom! parent nuclide daughter nuclide alpha particle Numbers must balance!! C. Johannesson

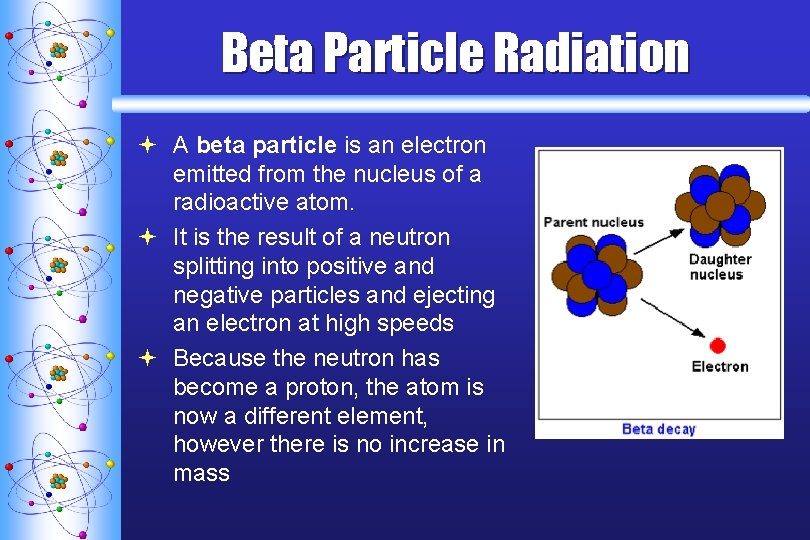
Beta Particle Radiation ª A beta particle is an electron emitted from the nucleus of a radioactive atom. ª It is the result of a neutron splitting into positive and negative particles and ejecting an electron at high speeds ª Because the neutron has become a proton, the atom is now a different element, however there is no increase in mass

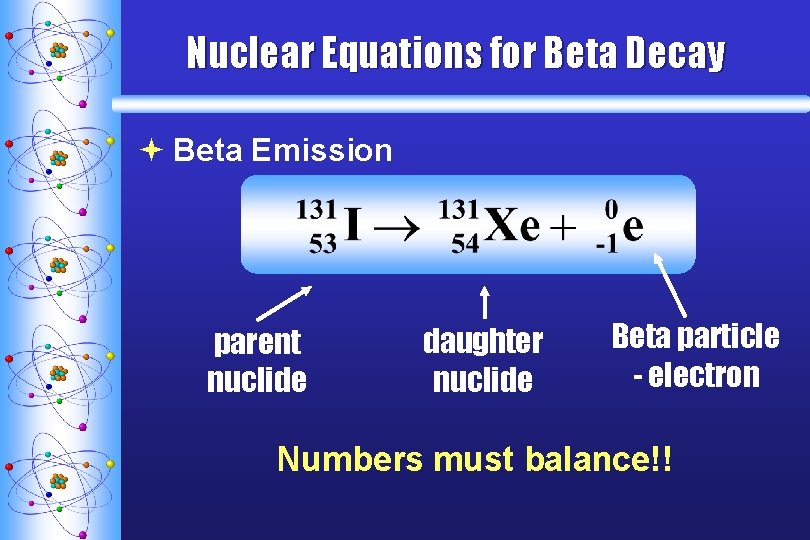
Nuclear Equations for Beta Decay ª Beta Emission parent nuclide daughter nuclide Beta particle - electron Numbers must balance!!

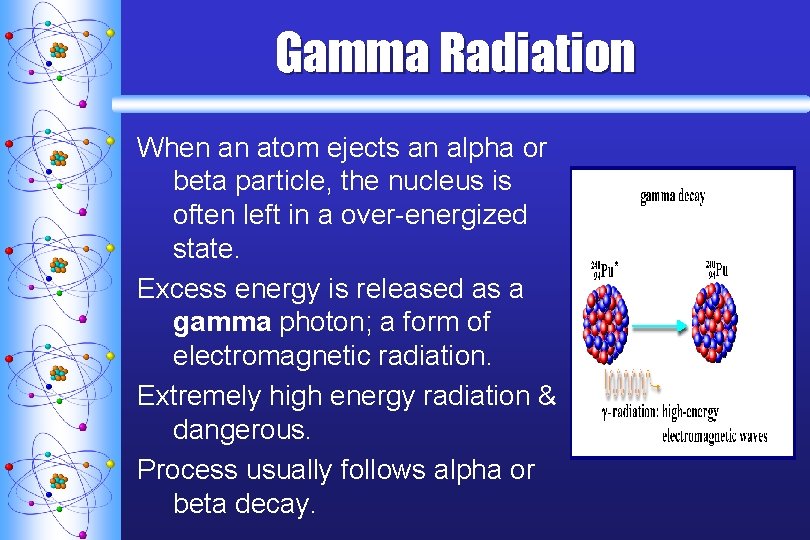
Gamma Radiation When an atom ejects an alpha or beta particle, the nucleus is often left in a over-energized state. Excess energy is released as a gamma photon; a form of electromagnetic radiation. Extremely high energy radiation & dangerous. Process usually follows alpha or beta decay.

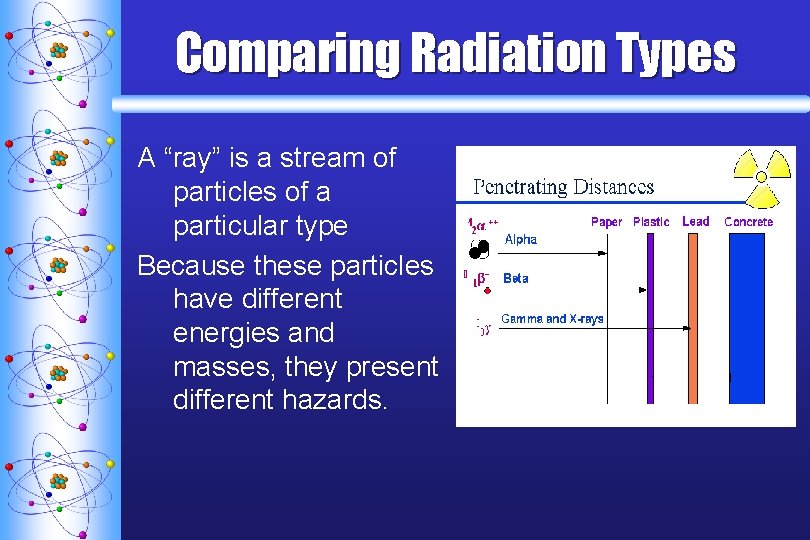
Comparing Radiation Types A “ray” is a stream of particles of a particular type Because these particles have different energies and masses, they present different hazards.

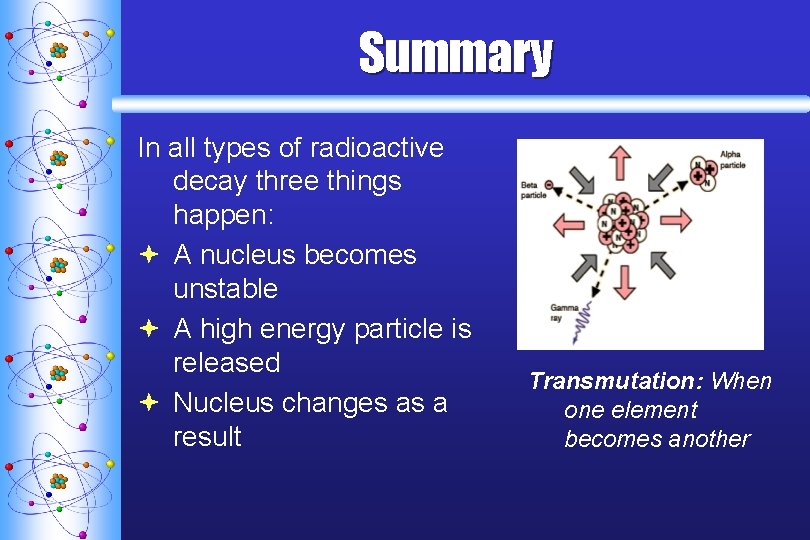
Summary In all types of radioactive decay three things happen: ª A nucleus becomes unstable ª A high energy particle is released ª Nucleus changes as a result Transmutation: When one element becomes another

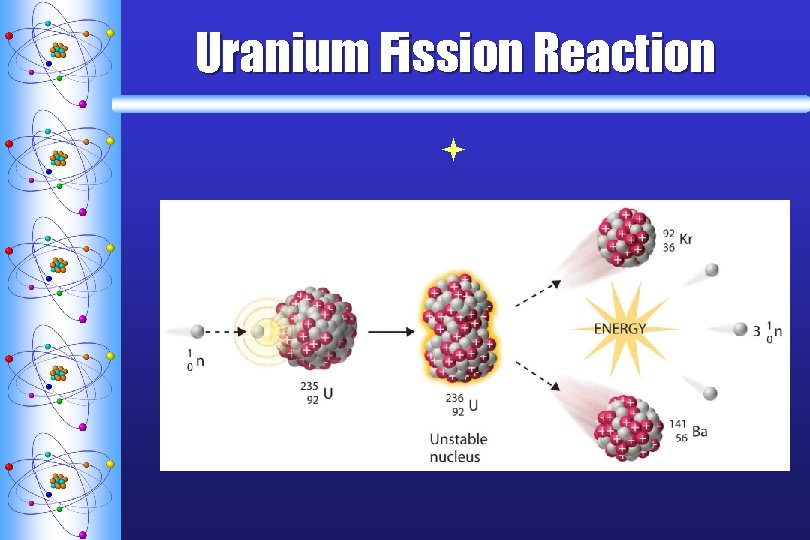
Fission ª Fission means divided. ª When a heavy nucleus is bombarded by neutrons the strong nuclear force weakens, but the electric repulsion remains strong. ª A fast moving neutron collides with the atom and splits it into smaller fragments ª High levels of energy released (7 million times the energy released by one TNT molecule!)

Uranium Fission Reaction ª

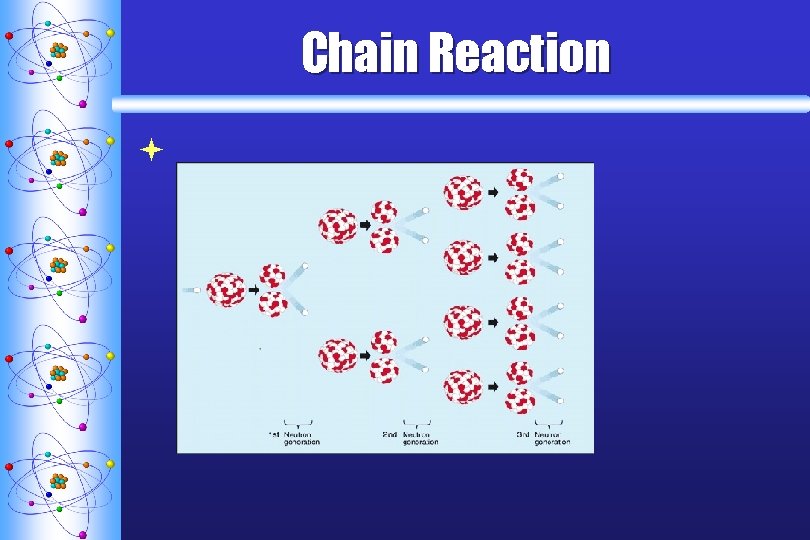
Chain Reaction ª

Fusion ª Nuclear Fusion is the opposite of fission ª When two lighter nuclei must travel at high speeds to overcome repulsion and form a heavier atom ª The hydrogen bomb is a destructive use of power from fusion.

Fusion ª

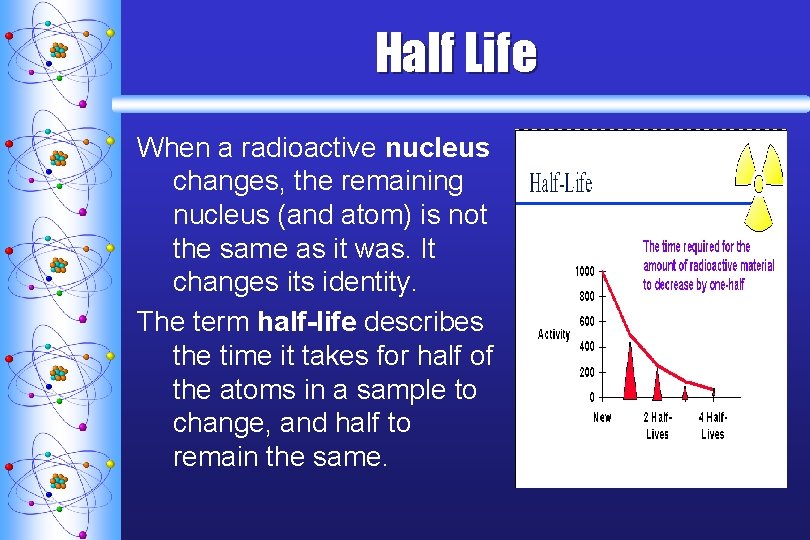
Half Life When a radioactive nucleus changes, the remaining nucleus (and atom) is not the same as it was. It changes its identity. The term half-life describes the time it takes for half of the atoms in a sample to change, and half to remain the same.

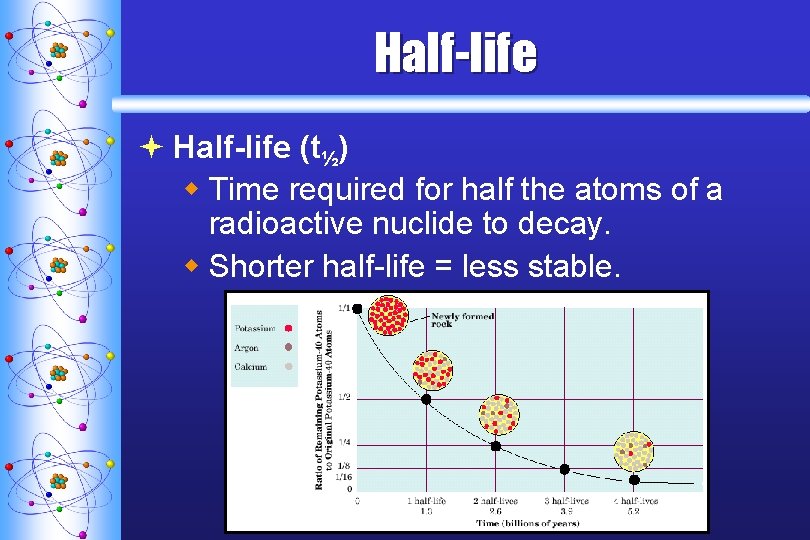
Half-life ª Half-life (t½) w Time required for half the atoms of a radioactive nuclide to decay. w Shorter half-life = less stable. C. Johannesson

Understanding Half Life 1. 2. 3. 4. Approximately what percentage of parent isotopes remains after 2 half-lives have passed? If a rock initially contained 10 milligrams of a radioactive parent when it first crystallized, how much remains after 4 half-lives? What % parent remains after 10 half-lives? If a mineral contains 1. 56% of its original parent isotopes, how many half-lives have passed? 25 % 0. 625 mg Assignment: Radioactive Decay Worksheet 0. 1% 6