Radical Chain Reactions Substitution Theory 1838 chlorination of

Radical Chain Reactions
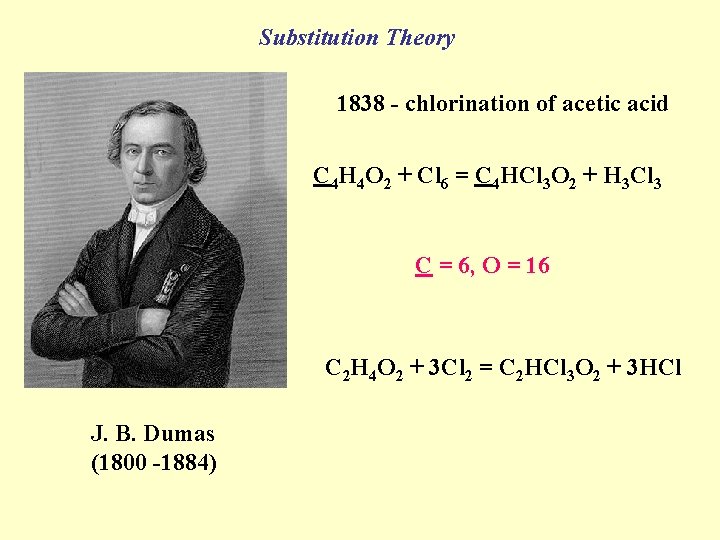
Substitution Theory 1838 - chlorination of acetic acid C 4 H 4 O 2 + Cl 6 = C 4 HCl 3 O 2 + H 3 Cl 3 C = 6, O = 16 C 2 H 4 O 2 + 3 Cl 2 = C 2 HCl 3 O 2 + 3 HCl J. B. Dumas (1800 -1884)
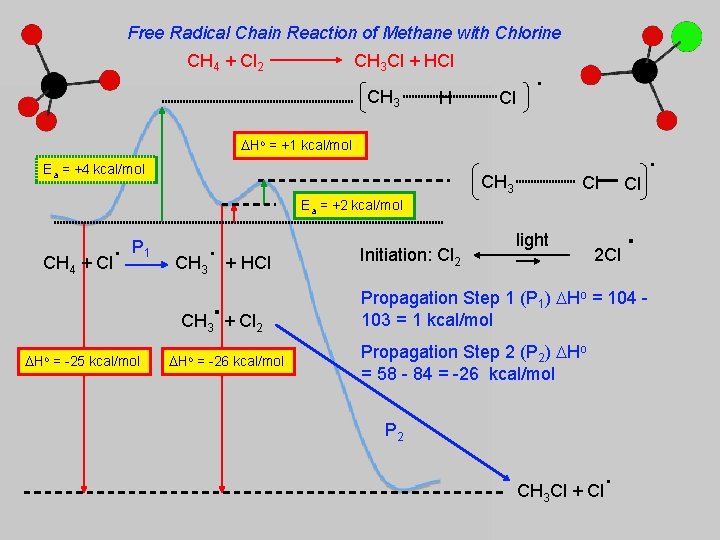
Free Radical Chain Reaction of Methane with Chlorine CH 4 + Cl 2 CH 3 Cl + HCl CH 3 H Cl . Ho = +1 kcal/mol Ea = +4 kcal/mol . CH 3 Cl Ea = +2 kcal/mol CH 4 . + Cl P 1 . CH + HCl 3 . CH 3 + Cl 2 Ho = -25 kcal/mol Ho = -26 kcal/mol Initiation: Cl 2 light 2 Cl Cl . Propagation Step 1 (P 1) Ho = 104 103 = 1 kcal/mol Propagation Step 2 (P 2) Ho = 58 - 84 = -26 kcal/mol P 2 . CH Cl + Cl 3
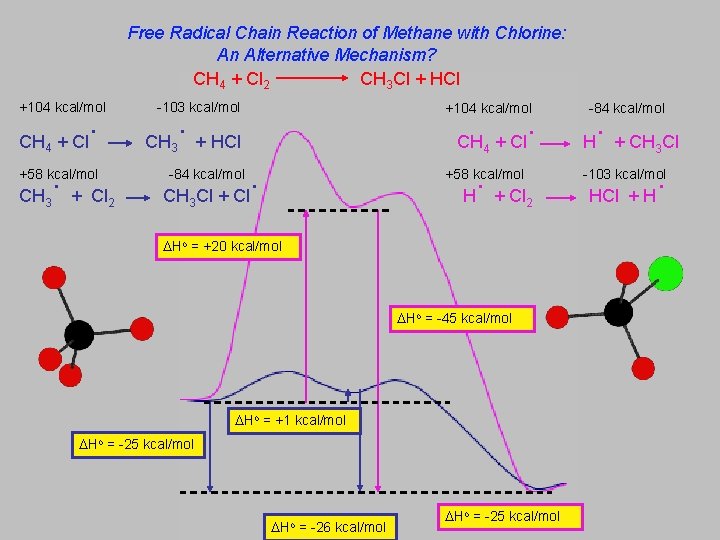
Free Radical Chain Reaction of Methane with Chlorine: An Alternative Mechanism? CH 4 + Cl 2 CH 3 Cl + HCl +104 kcal/mol CH 4 + Cl . CH . +58 kcal/mol 3 + Cl 2 -103 kcal/mol +104 kcal/mol . CH 3 + HCl CH 4 . CH Cl + Cl . H + Cl -84 kcal/mol +58 kcal/mol 3 . H + CH Cl 3 . -103 kcal/mol 2 Ho = +20 kcal/mol Ho = -45 kcal/mol Ho = +1 kcal/mol Ho = -25 kcal/mol Ho = -26 kcal/mol -84 kcal/mol Ho = -25 kcal/mol HCl + H
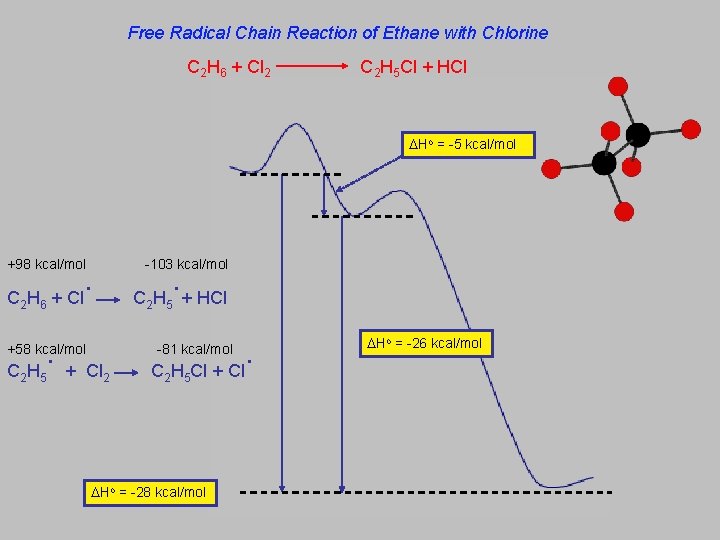
Free Radical Chain Reaction of Ethane with Chlorine C 2 H 6 + Cl 2 C 2 H 5 Cl + HCl Ho = -5 kcal/mol +98 kcal/mol C 2 H 6 . + Cl . CH +58 kcal/mol 2 5 -103 kcal/mol . C H + HCl 2 5 . C H Cl + Cl -81 kcal/mol + Cl 2 2 5 Ho = -28 kcal/mol Ho = -26 kcal/mol
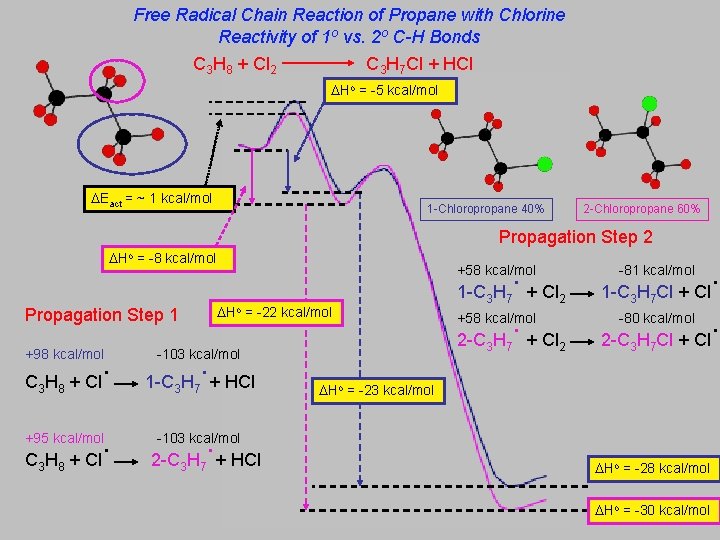
Free Radical Chain Reaction of Propane with Chlorine Reactivity of 1 o vs. 2 o C-H Bonds C 3 H 8 + Cl 2 C 3 H 7 Cl + HCl Ho = -5 kcal/mol Eact = ~ 1 kcal/mol 1 -Chloropropane 40% 2 -Chloropropane 60% Propagation Step 2 Ho = -8 kcal/mol Ho Propagation Step 1 . + Cl +98 kcal/mol C 3 H 8 . + Cl +95 kcal/mol C 3 H 8 . 1 -C H + Cl +58 kcal/mol = -22 kcal/mol . 1 -C H + HCl 7 7 . 2 -C H + Cl 2 +58 kcal/mol 3 -103 kcal/mol 3 3 -81 kcal/mol 7 1 -C 3 H 7 Cl + Cl -80 kcal/mol 2 2 -C 3 H 7 Cl + Cl Ho = -23 kcal/mol . -103 kcal/mol 2 -C 3 H 7 + HCl Ho = -28 kcal/mol Ho = -30 kcal/mol . .
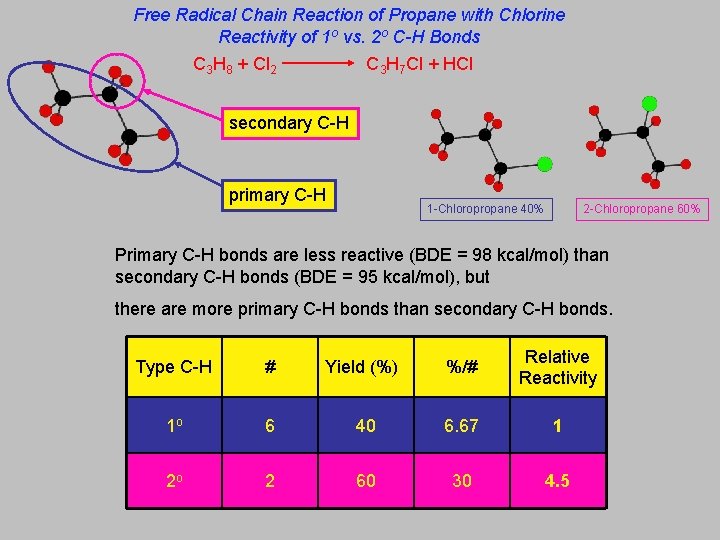
Free Radical Chain Reaction of Propane with Chlorine Reactivity of 1 o vs. 2 o C-H Bonds C 3 H 8 + Cl 2 C 3 H 7 Cl + HCl secondary C-H primary C-H 1 -Chloropropane 40% 2 -Chloropropane 60% Primary C-H bonds are less reactive (BDE = 98 kcal/mol) than secondary C-H bonds (BDE = 95 kcal/mol), but there are more primary C-H bonds than secondary C-H bonds. Type C-H # Yield (%) %/# Relative Reactivity 1 o 6 40 6. 67 1 2 o 2 60 30 4. 5
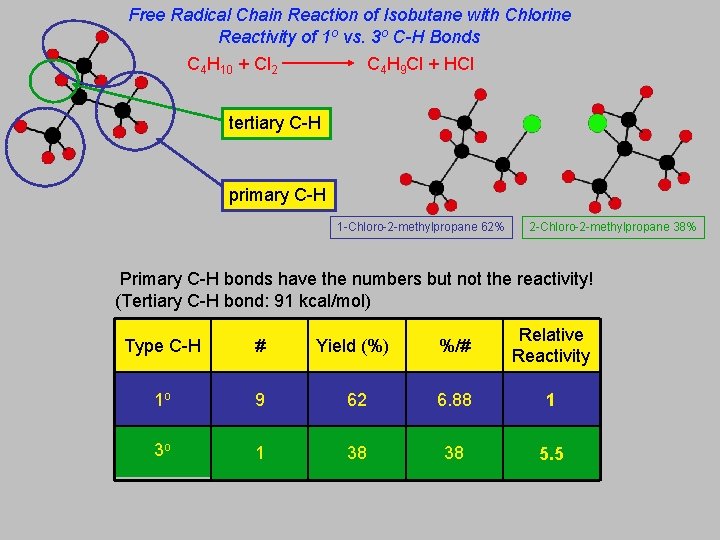
Free Radical Chain Reaction of Isobutane with Chlorine Reactivity of 1 o vs. 3 o C-H Bonds C 4 H 10 + Cl 2 C 4 H 9 Cl + HCl tertiary C-H primary C-H 1 -Chloro-2 -methylpropane 62% 2 -Chloro-2 -methylpropane 38% Primary C-H bonds have the numbers but not the reactivity! (Tertiary C-H bond: 91 kcal/mol) Type C-H # Yield (%) %/# Relative Reactivity 1 o 9 62 6. 88 1 3 o 1 38 38 5. 5
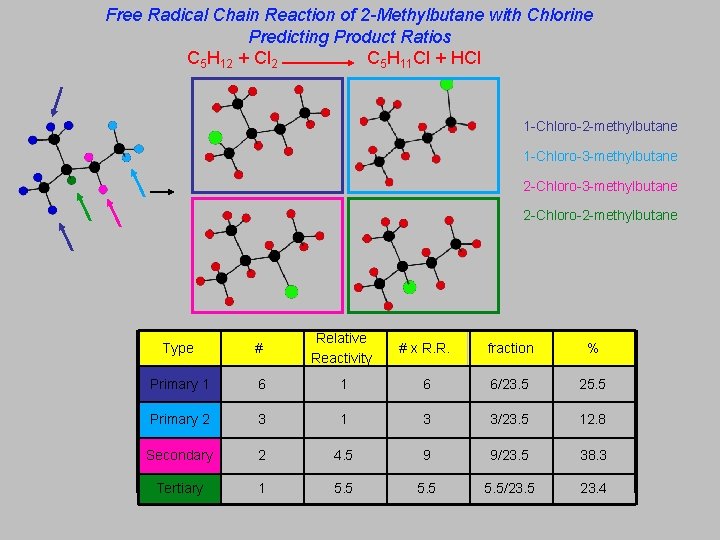
Free Radical Chain Reaction of 2 -Methylbutane with Chlorine Predicting Product Ratios C 5 H 12 + Cl 2 C 5 H 11 Cl + HCl 1 -Chloro-2 -methylbutane 1 -Chloro-3 -methylbutane 2 -Chloro-2 -methylbutane Primary 1 Primary 2 Type # Relative Reactivity # x R. R. fraction % Secondary Primary 1 6 6/23. 5 25. 5 Primary 2 3 1 3 3/23. 5 12. 8 Secondary 2 4. 5 9 9/23. 5 38. 3 Tertiary 1 5. 5/23. 5 23. 4 Tertiary
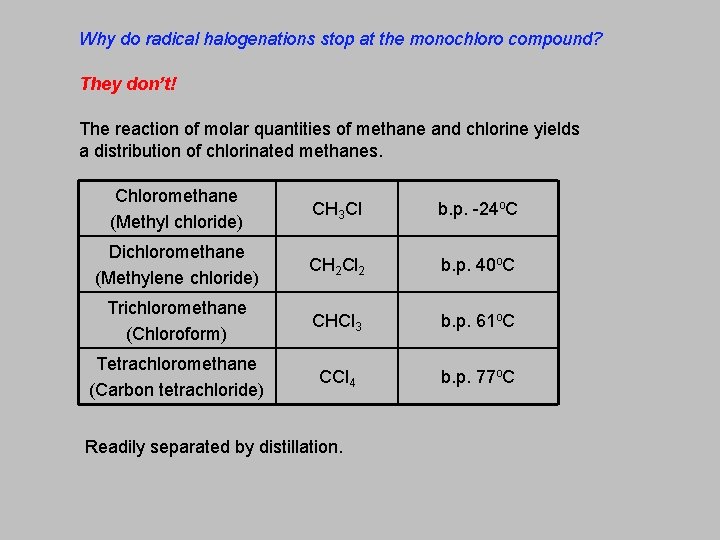
Why do radical halogenations stop at the monochloro compound? They don’t! The reaction of molar quantities of methane and chlorine yields a distribution of chlorinated methanes. Chloromethane (Methyl chloride) CH 3 Cl b. p. -24 o. C Dichloromethane (Methylene chloride) CH 2 Cl 2 b. p. 40 o. C Trichloromethane (Chloroform) CHCl 3 b. p. 61 o. C Tetrachloromethane (Carbon tetrachloride) CCl 4 b. p. 77 o. C Readily separated by distillation.
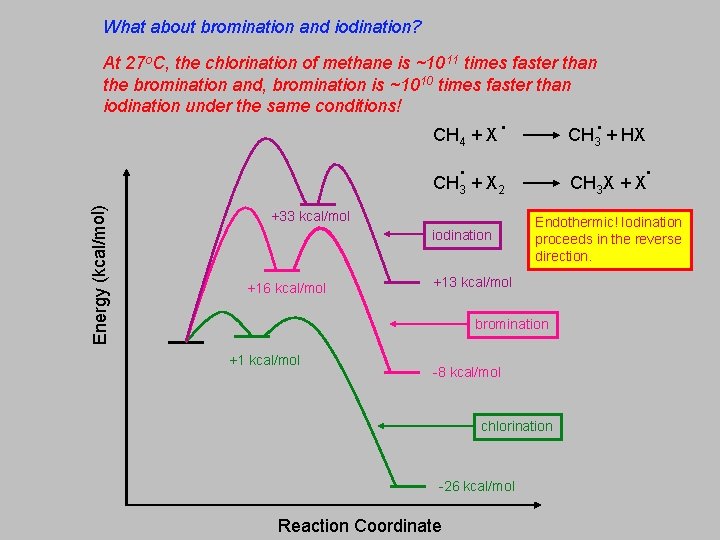
What about bromination and iodination? At 27 o. C, the chlorination of methane is ~1011 times faster than the bromination and, bromination is ~1010 times faster than iodination under the same conditions! CH 4 + X . CH + X Energy (kcal/mol) 3 . . CH X + X 2 +33 kcal/mol iodination +16 kcal/mol . CH 3 + HX 3 Endothermic! Iodination proceeds in the reverse direction. +13 kcal/mol bromination +1 kcal/mol -8 kcal/mol chlorination -26 kcal/mol Reaction Coordinate
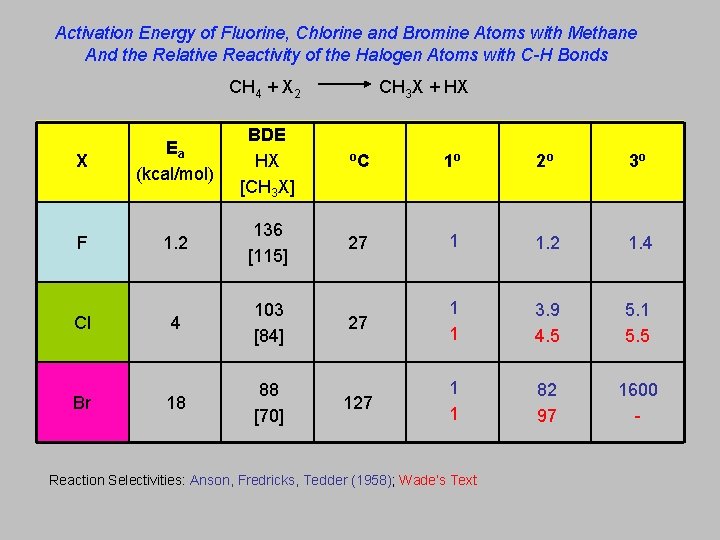
Activation Energy of Fluorine, Chlorine and Bromine Atoms with Methane And the Relative Reactivity of the Halogen Atoms with C-H Bonds CH 4 + X 2 X F Cl Br CH 3 X + HX Ea (kcal/mol) BDE HX [CH 3 X] o. C 1 o 2 o 3 o 1. 2 136 [115] 27 1 1. 2 1. 4 4 103 [84] 27 1 1 3. 9 4. 5 5. 1 5. 5 18 88 [70] 127 1 1 82 97 1600 - Reaction Selectivities: Anson, Fredricks, Tedder (1958); Wade’s Text
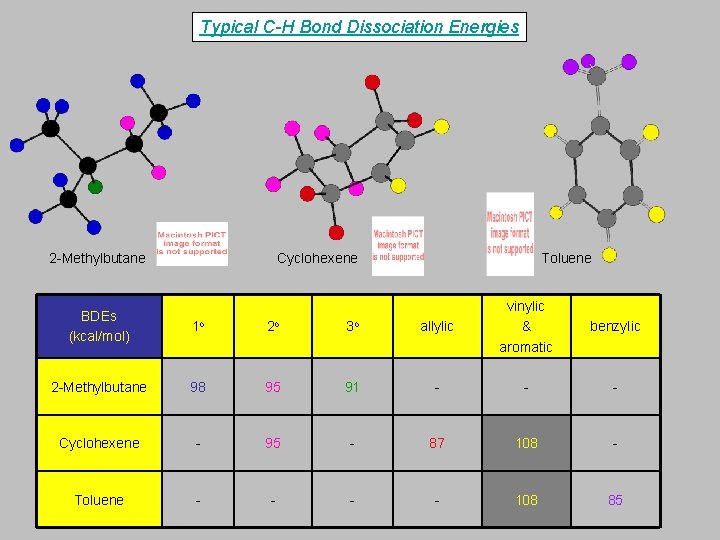
Typical C-H Bond Dissociation Energies 2 -Methylbutane Cyclohexene Toluene allylic vinylic & aromatic benzylic 91 - - - 95 - 87 108 - - 108 85 BDEs (kcal/mol) 1 o 2 o 3 o 2 -Methylbutane 98 95 Cyclohexene - Toluene -

Allylic Bromination 1 st Propagation Step 2 nd Propagation Step Generation of Br 2 in low concentration Allylic Bromination rate = kallylic[alkene][Br 2] Addition of Br 2 to the double bond N-Bromosuccinimide (more dense than CCl 4) Succinimide (less dense than CCl 4) rate = kaddn[alkene][Br 2]2

The End F. E Ziegler 2009
- Slides: 15