Radiant Energy Much of the understanding of how























- Slides: 28

Radiant Energy: • Much of the understanding of how electrons behave in atoms comes from studies of how light interacts with matter. • Light travels through space and is a form of radiant energy. • It is this energy that causes you to feel hot when you stand in sunlight. • But, how does light carry energy through space?

Waves: • ___________________Consists of electric and magnetic fields oscillating at right angles to each other and to the direction of motion of the wave. • Examples:

Waves: • ___________The height of a wave measured from its origin to its crest. The brightness, or intensity of light depends on the amplitude of light.

Waves: • __________the distance between successive crests of the wave. Visible light, or the light you see with your eyes has a wavelength of 400 -750 nanometers. You perceive light because of chemical reactions in your eyes.

Waves: • ___________tells how fast the wave oscillates up and down. The frequency of light is measured by the number of times a light wave completes a cycle of upward and downward motion in one second. Radio stations operate on frequencies.

Waves: • ____________Light moves through space at a speed of 3. 0 X 10⁸ m/s. • Because light moves at a constant speed, there is a relationship between its wavelength and its frequency. • The shorter the wavelength, the shorter the frequency. This relationship can be expressed in an equation.

Speed of light. • The speed of light can be represented by the equation: • ___________ • Where • λ = (the Greek letter lambda), stands for )__________ • v = (the Greek letter for nu), stands for ___________ • c = the speed of light

Math tip: • Frequency and wavelength of electromagnetic radiation are inversely proportional. • _______________ • Thus, high frequency radiation has short wavelengths whereas long wavelength radiation has low frequencies.

Visible Spectrum: • The array of colors that we are able to see is called the______________________ • The visible spectrum is an example of a continuous spectrum because one color fades into the next. • The different colors have different wavelengths (and therefore different frequencies). • Violet has the shortest wavelength (and the highest frequency). Red has the longest wavelength (and the shortest frequency).

Planck’s Theory: • In 1900, the German physicist Max Planck (1858 -1947) was able to predict accurately how the spectrum of radiation emitted by an object changes with its temperature. • ___________ Proposed that there is a fundamental restriction on the amounts of energy that an object emits or absorbs, and he called each of these pieces of energy a quantum. • Quantum means a fixed amount.

Planck’s Theory: • Planck’s equation relates the amount of energy, E, to the frequency, v, of the radiation. • ___________ • h is a constant known as Planck’s constant and has a value of 6. 6262 x 10⁻³⁴ J-s (Joules per second)

Planck’s Theory: • Planck’s Theory proposes that the energies absorbed or emitted by atoms are quantized, which means that their values are restricted to certain quantities. • Example: If a car were quantized, it would only be able to move at certain speeds. So, if the car’s fundamental quantum of energy corresponds to a speed of 10 km/hr, and the car has 7 quanta of energy, it will move at a speed of 70 km/hr.

The Photoelectric Effect: • In the _______________, electrons are ejected from a surface of a metal when light shines on the metal.

The Photoelectric effect: • The operation of many electronic devices such as photocells in camera light meters , is based on the photoelectric effect. • For each metal, a minimum frequency of light is needed to release electrons. • Example:

Photoelectric Effect: Photons • Einstein proposed that light consists of quanta of energy that behave like tiny particles of light. • He called this energy quanta _____________.

Photoelectric Effect: • What is important is the energy (and thus the frequency) of the photon, not the number of photons (the intensity of the light). • So, why does violet light free electrons from sodium metal but red light does not? • _________________________________________________________.

Applications of the Photoelectric Effect: • The relationship between the frequency of light and the energy of a photon helps to explain some of the effects of different kinds of electromagnetic radiation. • Example: . _______ have high frequencies. • Thus their photons have high energies – high enough to be capable of damaging organisms. • ___________on the other hand, have low frequencies. • Their photons have low frequencies and therefore do not pose a health risk.

Dual Nature of Radiant Energy: • The idea that light consists of tiny particles, or photons, was proven in 1923 when the American Physicist _________(1892 -1962) demonstrated that a photon can collide with an electron. • Thus, photons behave like particles, but a very special particle that always travels at the speed of light and has an associated frequency and wavelength. • _____________________________.

Line Spectra: • A spectrum that contains only certain colors, or wavelengths, is called a ______________________

Line Spectra: • The atoms must somehow absorb energy and then give the energy off in the form of light. • For every element, the emitted light contains only certain wavelengths, giving each element a unique line spectrum. • The line spectrum is also referred to as the ______________________of an element.

Line Spectra: • Example:

The Bohr model of the hydrogen atom: • Danish Physicist _________________(1885 -1962) was the first scientist to see the connection between the wavelengths an element emits and its atomic structure. • Bohr realized that Planck’s idea of quantization could be applied to the model to explain the line spectra of elements.

The Bohr model of the hydrogen atom: • In terms of Rutherford’s Planetary model, this means that the electron is allowed to have only certain orbits corresponding to certain amounts of energy. • Bohr labeled each energy level, and consequently each orbit, by a ___________________________

The Bohr model of the hydrogen atom: • For the lowest energy level, or ground state, n = 1. • The energy level corresponds to the orbit closest to the nucleus. • When the electron absorbs the appropriate amount of energy, it jumps to the level of higher energy, called the ______________. • The excited states have ____________________and so forth. • The excited states represent larger orbits with the electron farther from the nucleus.

Matter Waves: • When light travels through space, it behaves like a wave. • When light interacts with matter, its behavior can be like that of a stream of particles. • In 1924, a French graduate student name _________________thought that if energy has a dual nature (both wavelengths and particles) then matter must also. • He reasoned that particles of matter should behave like waves and exhibit a wavelength, just like waves of light behave like particles of matter.

Matter Waves: • De Broglie referred to the wavelike behavior of particles as ______________________.

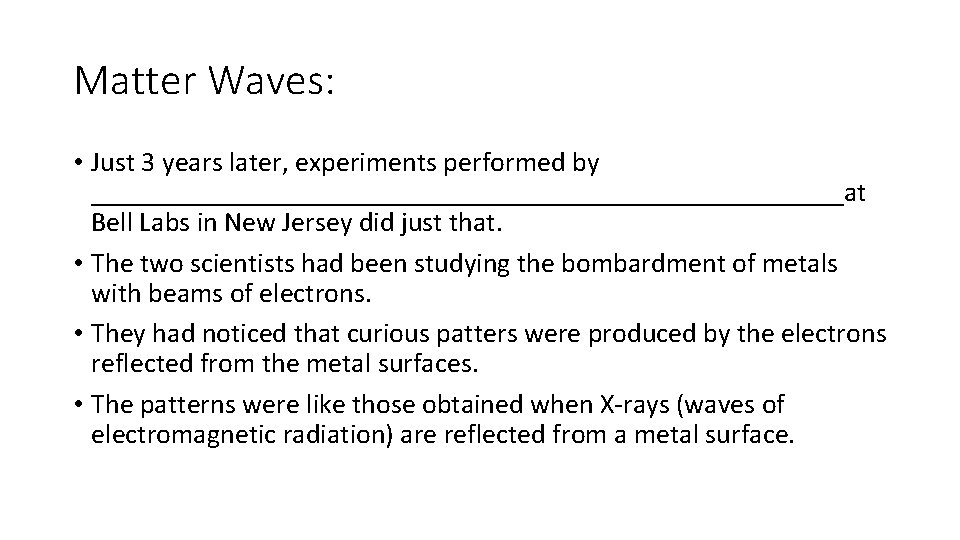
Matter Waves: • Just 3 years later, experiments performed by ___________________________at Bell Labs in New Jersey did just that. • The two scientists had been studying the bombardment of metals with beams of electrons. • They had noticed that curious patters were produced by the electrons reflected from the metal surfaces. • The patterns were like those obtained when X-rays (waves of electromagnetic radiation) are reflected from a metal surface.

Heisenburg’s Uncertainty Principle: • In 1927, _______________proposed his uncertainty principle, which states that the position and the momentum of a moving object cannot simultaneously be measured and known exactly.