Quiz 929 1000 g 1 n 1000 g




- Slides: 7

Quiz 9/29 1000 g = 1 ___ n 1000 g = 1 kg 2. 100 L = 1 ___ n 100 L = 1 HL 3. 23. 5 m = 2. 35 n 23. 5 m = 2. 35 Dam ___ n Match the unit with the measurement Density the measurement n g = mass a) g n L = volume Volume 3 = density b) L n g/cm Mass c) g/cm 3 Number 5 -9. Write 3 physical properties of the items in the tubes a -> e 1.

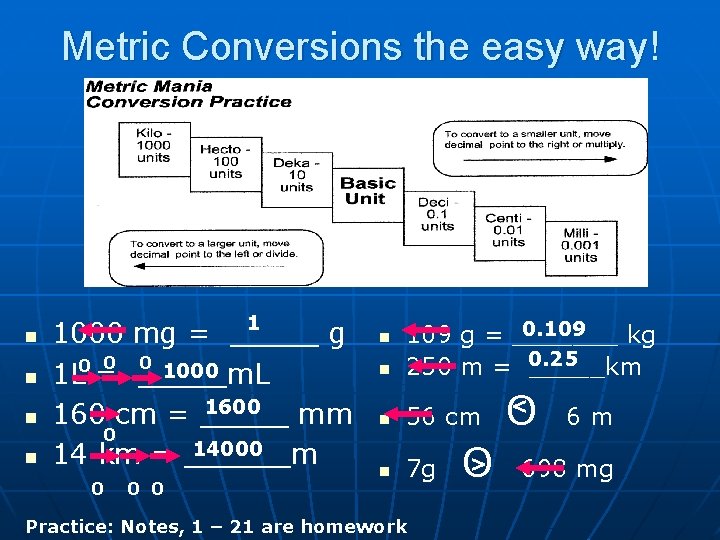
Metric Conversions the easy way! n n n 1 1000 mg = _____ g 0 0 1000 1 L 0 = _____m. L 1600 160 cm = _____ mm 0 14000 14 km = ______m 0 0 0 n 0. 109 g = _______ kg 250 m = 0. 25 _____km n 56 cm n 7 g n Practice: Notes, 1 – 21 are homework O < n < O 6 m 698 mg

Density: how much mass per unit volume? n n n Volume is measured in three dimensional quantities: m. L, L, cm 3, … Mass is measured in g, kg, … Density is a “derived unit” of g/ml or g/cm 3 or g/L or kg/L, …. Mass = Density x Volume Density = Mass Volume = Mass Density

Density = mass/volume n What is the density of a piece of concrete that has a mass of 8. 76 g and a volume of 3. 07 cm 3 Density = Mass Volume mass = 8. 76 g density = ? Mass = Density x Volume = Mass Density Volume = 3. 07 cm 3 density = 8. 76 g 3. 07 cm 3 = 2. 85 g/cm 3

Density = mass/volume n Limestone has a density of 2. 72 g/cm 3. What is the mass of 24. 9 cm 3 of limestone? Density = Mass Volume mass = ? density = 2. 72 g/cm 3 Mass = Density x Volume = Mass Density Volume = 24. 9 cm 3 mass = (2. 72 g/cm 3)(24. 9 cm 3) = 67. 7 g

Density Practice for extra credit. 1. 2. 3. Illegal ivory is sometimes detected on the basis of density. What is the density of a sample of ivory whose volume is 14. 5 cm 3 and whose mass is 26. 8 g? An archeologist finds that a piece of pottery has a mass of 0. 61 g and a volume of 0. 26 cm 3. What is the density of the pottery? Calcium chloride is used as a de-icer on roads in winter. It has a density of 2. 50 g/cm 3. What is the volume of 7. 91 g of this substance?

Key Terms: 15 points, Turn in Today n n n n n Mass (kg) Weight (lbs) Volume (ml or L) balance (g) length (cm or m) time (sec) temperature (o. C or K) Accuracy Precision n n Percent error Significant Digits Derived units density (g/cm 3) or • (g/ml) n n Find the definition for each, write a sentence using the word correctly. counting number prefix • • kilo deci milli micro