Quiz 5 1 1 In a chemical equation



















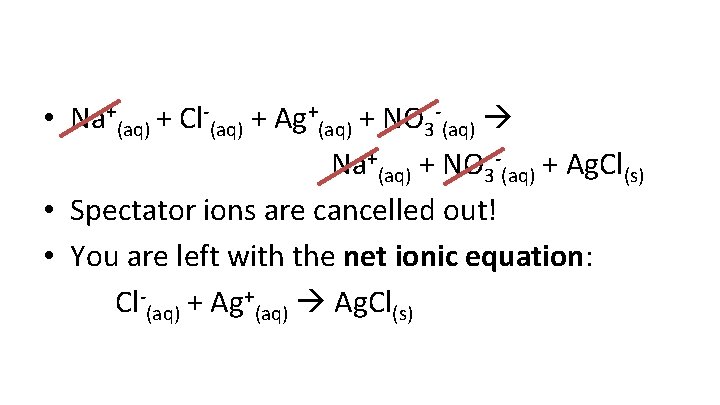


- Slides: 29

Quiz 5. 1 1. In a chemical equation, “(aq)” means “aqueous”. What does this mean? 2. Balance the following: __Al 2 O 3 __Al + __O 2 3. Write the correct formula for sodium nitrate 4. Write the correct formula for carbonic acid

Quiz 5. 1 1. In a chemical equation, “(aq)” means “aqueous”. What does this mean? 2. Balance the following: __Al 2 O 3 __Al + __O 2 3. Write the correct formula for sodium sulfide 4. Write the correct formula for sulfuric acid


5. 2 Types of Reactions How do you write net ionic equations?

Review

• When the identity of a substance changes, a chemical reaction has occurred • Atoms are being reorganized by breaking and creating bonds • Reactants Products

5 major chemical reaction types 1. Combustion 2. Synthesis Reaction 3. Decomposition reaction 4. Single replacement reaction 5. Double replacement reaction

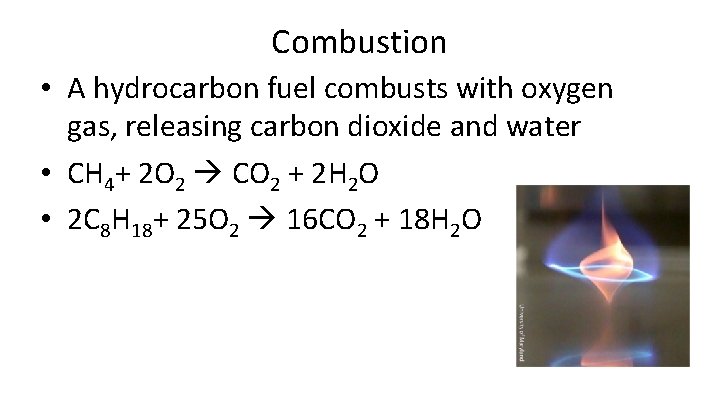
Combustion • A hydrocarbon fuel combusts with oxygen gas, releasing carbon dioxide and water • CH 4+ 2 O 2 CO 2 + 2 H 2 O • 2 C 8 H 18+ 25 O 2 16 CO 2 + 18 H 2 O

Synthesis reaction • Elements/compounds combine to form a new compound • A + B AB • 3 H 2(g) + N 2(g) 2 NH 3(g)

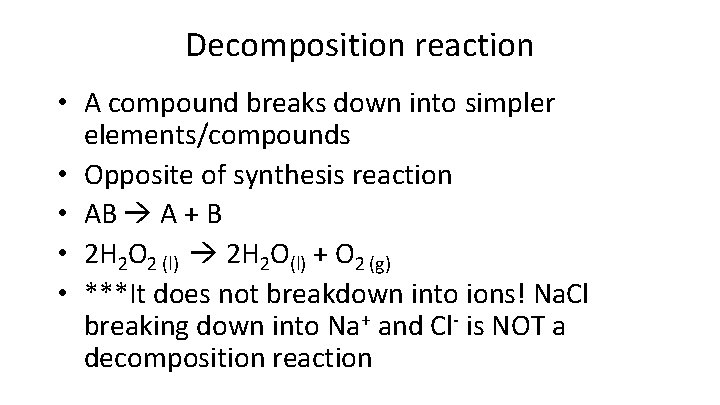
Decomposition reaction • A compound breaks down into simpler elements/compounds • Opposite of synthesis reaction • AB A + B • 2 H 2 O 2 (l) 2 H 2 O(l) + O 2 (g) • ***It does not breakdown into ions! Na. Cl breaking down into Na+ and Cl- is NOT a decomposition reaction

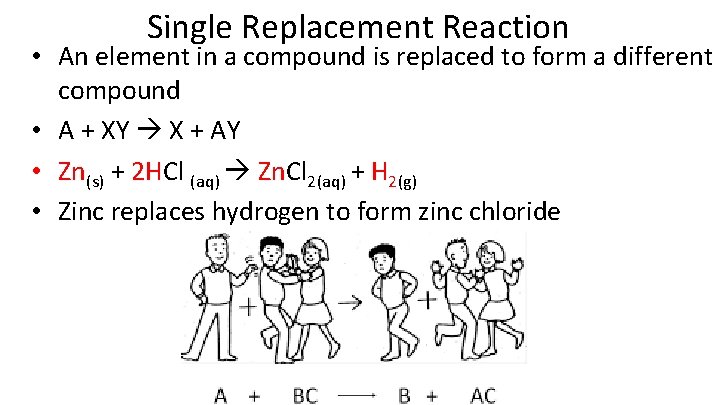
Single Replacement Reaction • An element in a compound is replaced to form a different compound • A + XY X + AY • Zn(s) + 2 HCl (aq) Zn. Cl 2(aq) + H 2(g) • Zinc replaces hydrogen to form zinc chloride

• Zn(s)+Fe. SO 4(aq)→Fe(s)+Zn. SO 4(aq) • Zinc replaces iron to form zinc sulfate

Activity Series • Single replacement reaction will only occur if the lone metal is more active than the metal in the compound • Zn(s) + 2 HCl (aq) Zn. Cl 2(aq) + H 2(g) • The zinc is higher on the list than hydrogen, making it more active; reaction will take place

• Fe(s)+Zn. SO 4(aq) → Zn(s)+Fe. SO 4(aq) • Identify iron and zinc on the activity series • Since zinc is more active, iron cannot take away the sulfate (SO 42 -) away from zinc • Reaction does NOT occur

Practice • KCl + Na • No reaction! • Mg + Fe. S Fe + Mg. S

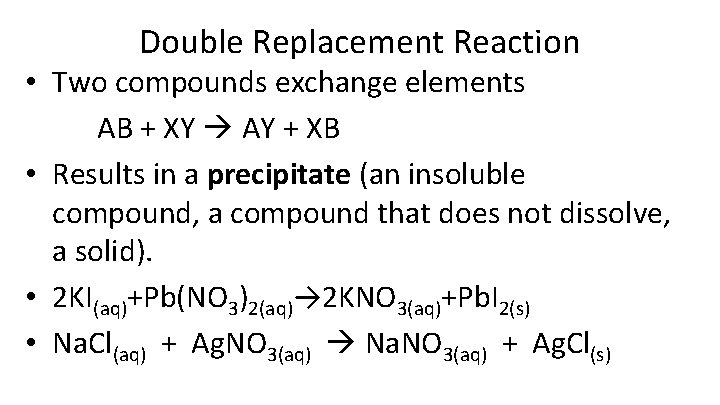
Double Replacement Reaction • Two compounds exchange elements AB + XY AY + XB • Results in a precipitate (an insoluble compound, a compound that does not dissolve, a solid). • 2 KI(aq)+Pb(NO 3)2(aq)→ 2 KNO 3(aq)+Pb. I 2(s) • Na. Cl(aq) + Ag. NO 3(aq) Na. NO 3(aq) + Ag. Cl(s)

What happens when things dissolve? • As salt dissolves, the salt breaks up into smaller ions. • Dissociation is the break up of these ions • The water surrounds individual ions due to the charges • Since the ions are split up individually, it seems to have “disappeared” • Does it really disappear?

• Na. Cl(aq) means that Na. Cl has been dissolved, and is separated into individual ions, Na+ and Cl-

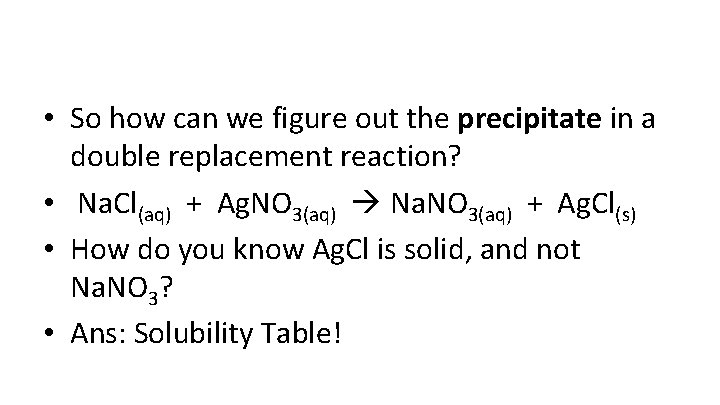
• So how can we figure out the precipitate in a double replacement reaction? • Na. Cl(aq) + Ag. NO 3(aq) Na. NO 3(aq) + Ag. Cl(s) • How do you know Ag. Cl is solid, and not Na. NO 3? • Ans: Solubility Table!

Solubility Table: on Website • If compound is insoluble, the compound must be written with (s) • If compound is soluble, the compound must be written with (aq) • Hint: Soluble means able to be dissolved

Practice: Soluble? • • Al. Br 3 Yes Pb. SO 4 No KSO 4 Yes Fe. PO 4 No

Writing Ionic Equations • Separate (aq) substances into their cations and anions • Ca. Cl 2(aq) = • Ca 2+(aq) + 2 Cl-(aq)

• • Mg(NO 3)2(aq)= Mg 2+(aq) + 2 NO 3 -(aq) Zn. SO 4(aq) = Zn 2+(aq) + SO 42 -(aq)

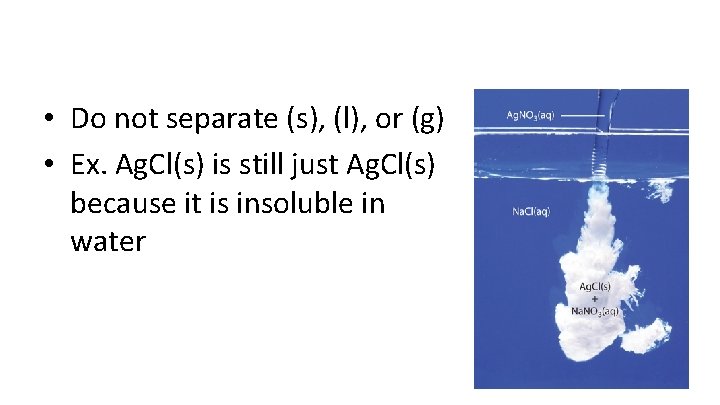
• Do not separate (s), (l), or (g) • Ex. Ag. Cl(s) is still just Ag. Cl(s) because it is insoluble in water

• So rewrite the following equation as an ionic equation • Na. Cl(aq) + Ag. NO 3(aq) Na. NO 3(aq) + Ag. Cl(s) • Na+(aq) + Cl-(aq) + Ag+(aq) + NO 3 -(aq) Na+(aq) + NO 3 -(aq) + Ag. Cl(s)

• Notice that Na+ and NO 3 - are the same in the reactant and product sides • Nothing is happening to these ions, and are called spectator ions • When spectator ions are removed from the ionic equation, the remaining ions make the net ionic equation
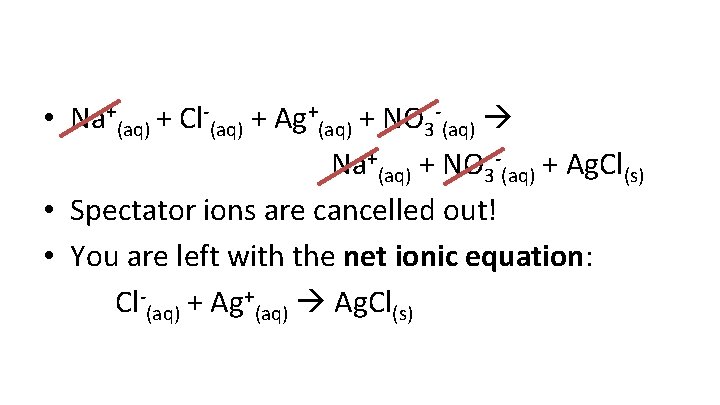
• Na+(aq) + Cl-(aq) + Ag+(aq) + NO 3 -(aq) Na+(aq) + NO 3 -(aq) + Ag. Cl(s) • Spectator ions are cancelled out! • You are left with the net ionic equation: Cl-(aq) + Ag+(aq) Ag. Cl(s)

Practice • Write out the ionic and net ionic equation of the following: • 2 KI(aq)+Pb(NO 3)2(aq)→ 2 KNO 3(aq)+Pb. I 2(s) • 2 K+(aq) + 2 I-(aq) + Pb 2+(aq) + 2 NO 3 -(aq) 2 K+(aq) + 2 NO 3 -(aq) + Pb. I 2(s) • Pb 2+(aq) + 2 I-(aq) Pb. I 2(s)

Categorize the following chemical reactions as single replacement, double replacement, combustion, combination, or decomposition. • Magnesium carbonate is heated strongly to produce magnesium oxide and carbon dioxide gas. • Solid potassium chlorate is heated to produce potassium chloride and oxygen gas.