Quiz 1 What is the oxidation number for

Quiz 1. What is the oxidation number for phosphorous in P 4 O 6 ? 2. If sulfur becomes an ion, what is its oxidation number? 3. What is the name of the following chemical? Ca. Br 2? 4. Write the following in the Stock method and Latin method: Fe. Br 3 5. What is the chemical formula for lithium sulfate?

GC Quiz 1. What is the oxidation number for phosphorous in P 4 O 6 ? 2. What is the oxidation number for magnesium in Mg. Cl 2 3. If sulfur becomes an ion, what is its oxidation number? 4. What is the name of the following chemical? Ca. Br 2? 5. What is the chemical formula for potassium nitride?

3. 2 Molecular Compounds (Covalent Compound)
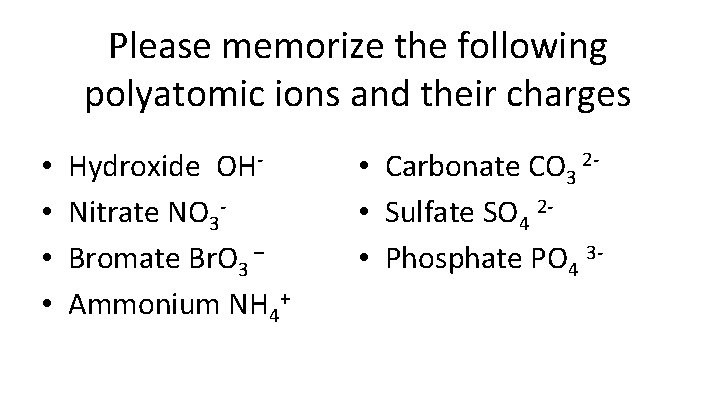
Please memorize the following polyatomic ions and their charges • • Hydroxide OHNitrate NO 3 Bromate Br. O 3 – Ammonium NH 4+ • Carbonate CO 3 2 • Sulfate SO 4 2 • Phosphate PO 4 3 -

• The most common polyatomic ion is simply “–ate” • Least amount of oxygen is “hypo—ite” • One more oxygen is “—ate” • One more oxygen is “per–ate”
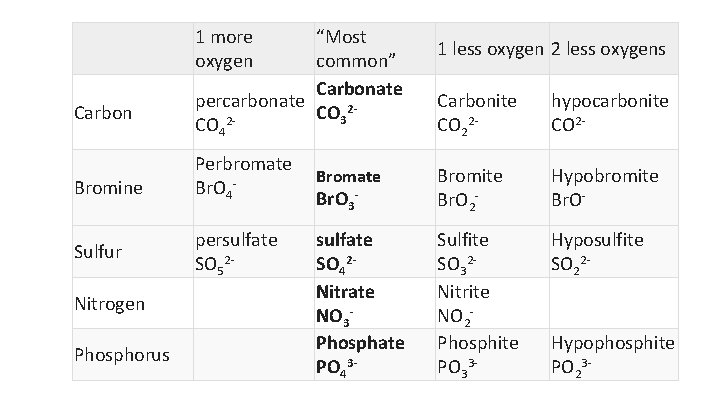
1 more oxygen Carbon Bromine Sulfur Nitrogen Phosphorus “Most common” Carbonate percarbonate CO 322 CO 4 Perbromate Br. O 4 persulfate SO 52 - 1 less oxygen 2 less oxygens Carbonite CO 22 - hypocarbonite CO 2 - Bromate Br. O 3 - Bromite Br. O 2 - Hypobromite Br. O- sulfate SO 42 Nitrate NO 3 Phosphate PO 43 - Sulfite SO 32 Nitrite NO 2 Phosphite PO 33 - Hyposulfite SO 22 - Hypophosphite PO 23 -
Molecular Compounds • Molecular compounds refer to compounds with covalent bonds • Covalent bonds form between two nonmetal elements • How do ionic compounds and molecular compounds differ?

• Is Li 2 O a molecular compound? • No! It’s ionic. Because it is formed from a metal and a nonmetal • What is it called? • Is N 2 O a molecular compound? • Yes! A covalent bond forms between the two nonmetal elements.
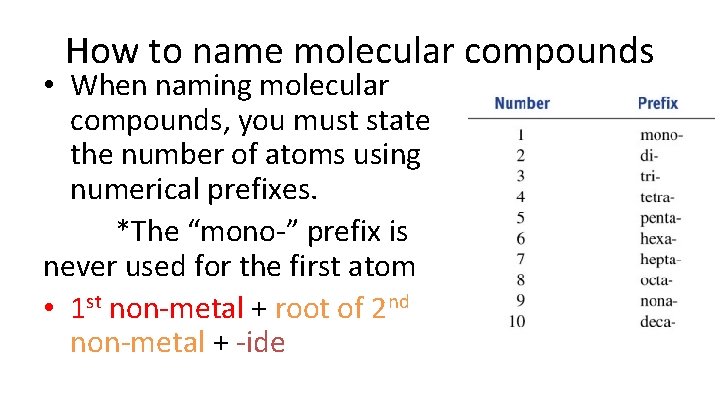
How to name molecular compounds • When naming molecular compounds, you must state the number of atoms using numerical prefixes. *The “mono-” prefix is never used for the first atom • 1 st non-metal + root of 2 nd non-metal + -ide

• • Examples NO Nitrogen monoxide (why not mononitrogen? ) N 2 O Dinitrogen monoxide S 2 Cl 2 Disulfur dichloride Cl 2 O 7 Dichlorine heptoxide

• • • BF 3 NO N 2 O 5 PCl 5 P 4 O 6 • • • Boron trifluoride Nitrogen monoxide Dinitrogen pentoxide Phosphorus pentachloride Tetraphosphorus hexoxide
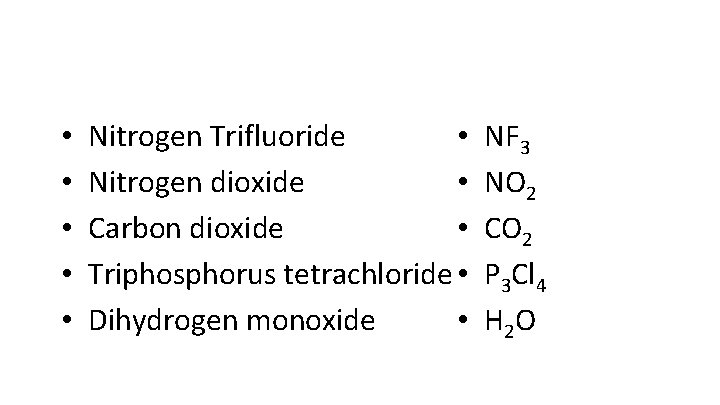
• • • Nitrogen Trifluoride • Nitrogen dioxide • Carbon dioxide • Triphosphorus tetrachloride • Dihydrogen monoxide • NF 3 NO 2 CO 2 P 3 Cl 4 H 2 O
Hydrates • Ionic crystals with a certain number of water molecules as part of its structures. Ex: • Name the ionic compound first, then add “hydrate” with proper Greek prefix to indicate number of water molecules

Practice • • • Ba. Cl 2 • 2 H 2 O Barium chloride dihydrate Na 2 CO 3 • 10 H 2 O Sodium carbonate decahydrate * Co. SO 4 • 6 H 2 O Cobalt (II) sulfate hexahydrate

Naming Acids • Acids are compounds that produce H+ ions • There are 2 methods to name acids 1) Acids that do not contain oxygen 2) Acids that do contain oxygen

Acids that do not contain oxygen • • Generally a binary acid (what does this mean? ) Hydro + root of non-metal + “ic” + acid HCl Hydrochloric acid H 2 S Hydrosulfuric acid (dihydrogen monosulfide can also work, and is considered a ‘common name’)

Acids that do contain oxygen • These are acids with polyatomic ions • Use the anion molecule to name acid, and add “acid” at end

• If anion ends with “–ate” add a “-ic” • If anion ends with “-ite” add a “-ous” • H 2 SO 4 The anion is SO 42 -, or sulfate • Sulfuric acid • H 2 SO 3 The anion is SO 32 -, or sulfite • Sulfurous acid • “Hydro-” is used on acids without oxygen only.
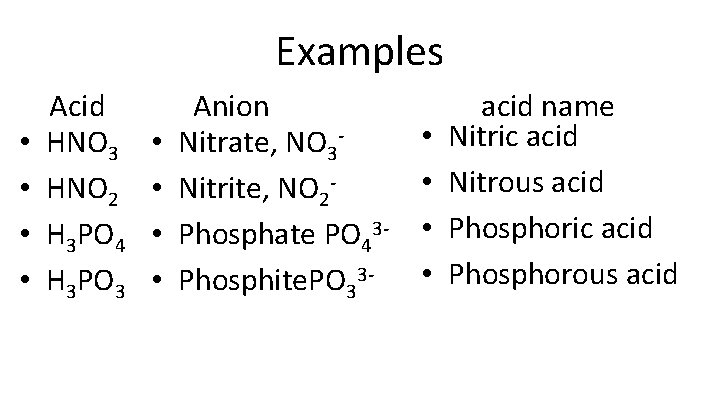
Examples • • Acid HNO 3 HNO 2 H 3 PO 4 H 3 PO 3 • • Anion Nitrate, NO 3 Nitrite, NO 2 Phosphate PO 43 Phosphite. PO 33 - • • acid name Nitric acid Nitrous acid Phosphoric acid Phosphorous acid

- Slides: 20