QUICK WRITE Question on page 30 Answer on















- Slides: 22

QUICK WRITE Question on page 30 Answer on page 29 WHEN YOU ARE THIRSTY, YOU NEED TO DRINK SOMETHING THAT IS MOSTLY WATER. WHY IS THE WATER YOU DRINK ABSOLUTELY NECESSARY?

Class Norms Ø Honor time limits Ø Actively Ø Listen Ø Place participate respectfully to your colleagues cell phones on vibrate or silent mode Ø Participants may write burning questions on a sticky note and place on the parking lot Ø BE PRESENT

Parking Lot Ø Burning Issues Ø Questions Ø Comments Ø Ideas to Share

Common Board Configuration DATE: August 23 -24, 2014 ESSENTIAL QUESTION: How do the unique properties of water allow life to exist on Earth? Objectives: Ø Explain how the shape of water molecule contributes to its unique qualities. Ø List and describe how each of the four properties of water make it essential for life on Earth. Home Learning: Ø Finish pages 20, 21, and 23 in your ISN

Agenda Ø Interactive Journal Review Setup Ø Water Properties Ø Complete Water Activity in ISN Ø Exit Ticket

Essential Question How do the unique properties of water allow life to exist on Earth?

Properties of Water http: //www. youtube. com/watch? v=HVT 3 Y 3_g. HGg

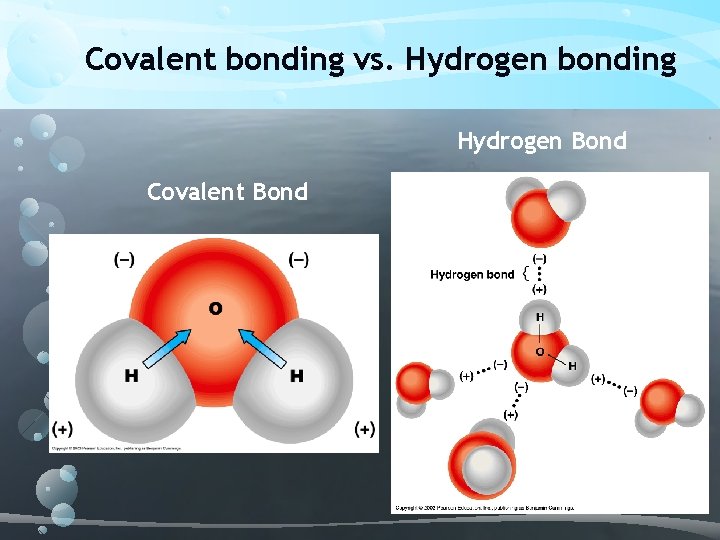
Covalent bonding Polar covalent bond – unequal sharing of electrons A great example of a molecule with polar covalent bonds is water. Why is water considered polar? What is a partial positive and partial negative charge?

Covalent bonding vs. Hydrogen bonding Hydrogen Bond Covalent Bond

Universal Solvent Water is the solvent of Life! Solute – substance dissolved in a solvent to form a solution Solvent – fluid that dissolves solutes Example: Ice Tea – water is the solvent and tea and sugar the solutes

Cohesion, Adhesion and Surface Tension Ø cohesion : water attracted to other water molecules because of polar properties Ø adhesion : water attracted to other materials Ø surface tension : water is pulled together creating the smallest surface area possible

Capillary Action Because water has both adhesive and cohesive properties, capillary action is present. Capillary Action = water’s adhesive property is the cause of capillary action. Water is attracted to some other material and then through cohesion, other water molecules move too as a result of the original adhesion. Ex: Think water in a straw Ex: Water moves through trees this way

High Heat Capacity In order to raise the temperature of water, the average molecular speed has to increase. It takes much more energy to raise the temperature of water compared to other solvents because hydrogen bonds hold the water molecules together! Water has a high heat capacity. “The specific heat is the amount of heat per unit mass required to raise the temperature by one degree Celsius. ”

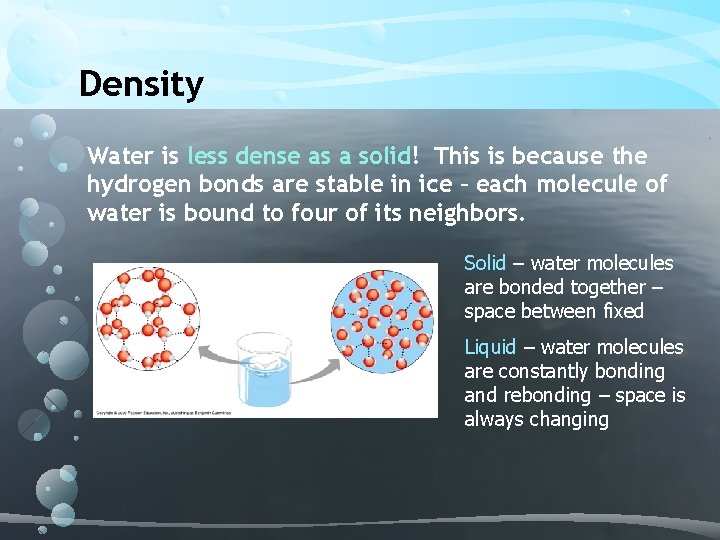
Density Water is less dense as a solid! This is because the hydrogen bonds are stable in ice – each molecule of water is bound to four of its neighbors. Solid – water molecules are bonded together – space between fixed Liquid – water molecules are constantly bonding and rebonding – space is always changing

So, can you name all of the properties of water? ü Adhesion ü Cohesion ü Capillary action ü High surface tension ü Holds heat to regulate temperature (High heat capacity) ü Less dense as a solid than a liquid

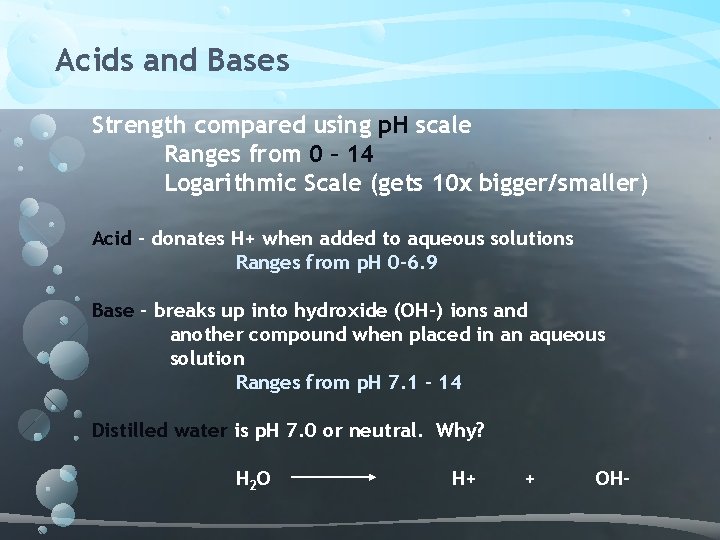
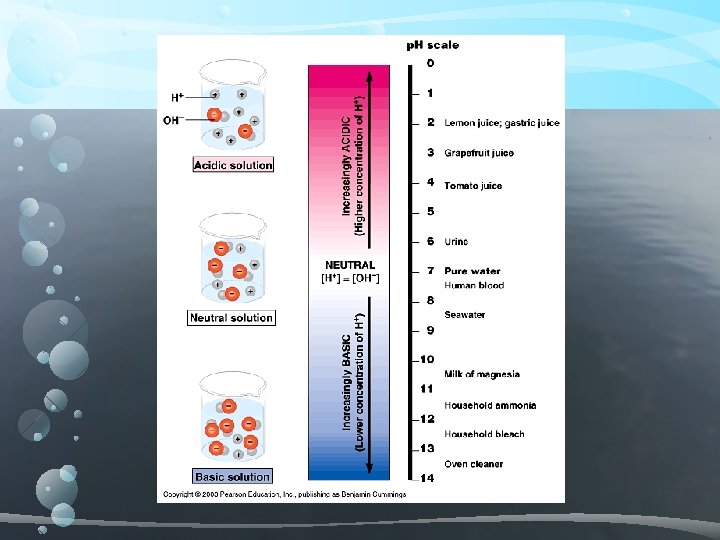
Acids and Bases Strength compared using p. H scale Ranges from 0 – 14 Logarithmic Scale (gets 10 x bigger/smaller) Acid – donates H+ when added to aqueous solutions Ranges from p. H 0 -6. 9 Base – breaks up into hydroxide (OH-) ions and another compound when placed in an aqueous solution Ranges from p. H 7. 1 – 14 Distilled water is p. H 7. 0 or neutral. Why? H 2 O H+ + OH-


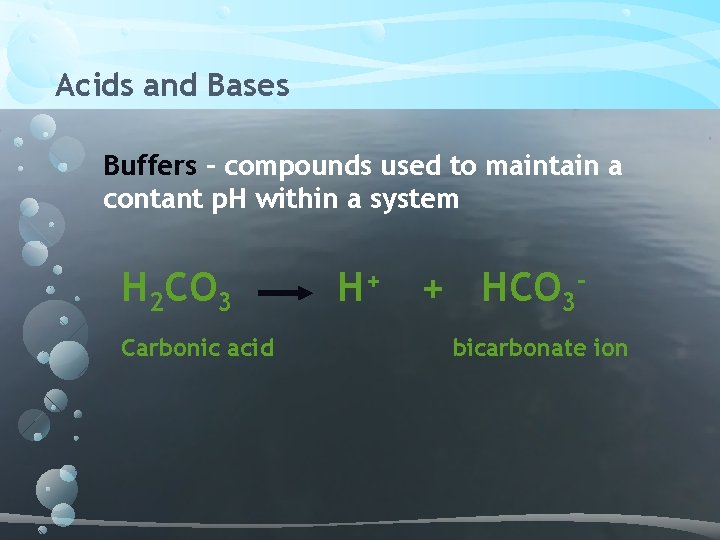
Acids and Bases Buffers – compounds used to maintain a contant p. H within a system H 2 CO 3 Carbonic acid H+ + HCO 3 bicarbonate ion

Acids and Bases

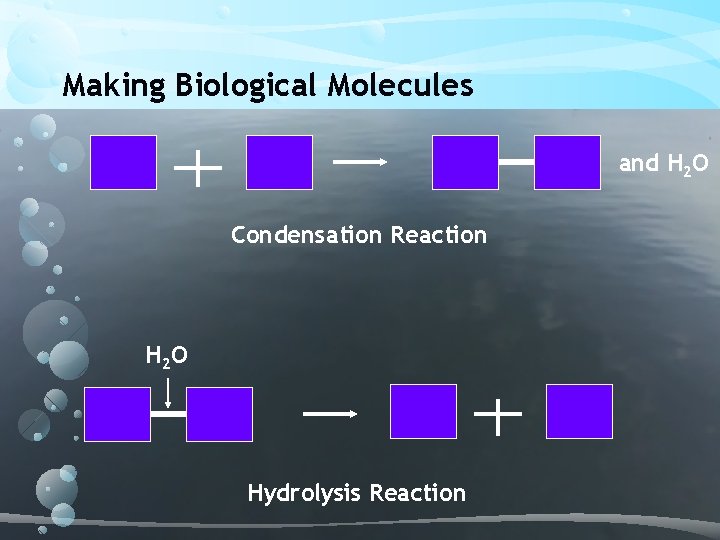
Making Biological Molecules and H 2 O Condensation Reaction H 2 O Hydrolysis Reaction

HOME LEARNING

“Small, Yes, But Mighty: The Molecule Called Water” (http: //www. nytimes. com/learning/teachers/featured_articles/20070710 tuesday. html), Discussion Questions 1) In what ways does life depend on water? 2) What does the article suggest about life on other planets? 3) How is water unusual for such a small molecule? 4) How is a hydrogen bond different from the covalent bonds that connect each water molecule’s atoms? 5) What properties of water can be directly attributed to the presence of hydrogen bonds between water molecules? 6) What differences in molecular structure might cause water to form hydrogen bonds while hydrogen sulfide does not form such bonds? 7) What conclusions can you draw about how lakes, oceans and the atmosphere would be affected if water had different freezing and boiling points? 8) Why do you think it is often cooler in the summer in areas close to lakes and oceans than farther inland? How does this account for our attraction to watery destinations?