Questions Why do some solids dissolve in water
























- Slides: 42

Questions • Why do some solids dissolve in water but others do not? • Why are some substances gases at room temperature, but others are liquid or solid? • What gives metals the ability to conduct electricity, what makes non-metals brittle? • The answers have to do with … Intermolecular forces

Intermolecular forces Overview • There are 2 types of attraction in molecules: intramolecular bonds & intermolecular forces • We have already looked at intramolecular bonds (ionic, polar, non-polar) • Intermolecular forces (IMF) have to do with the attraction between molecules (vs. the attraction between atoms in a molecule)

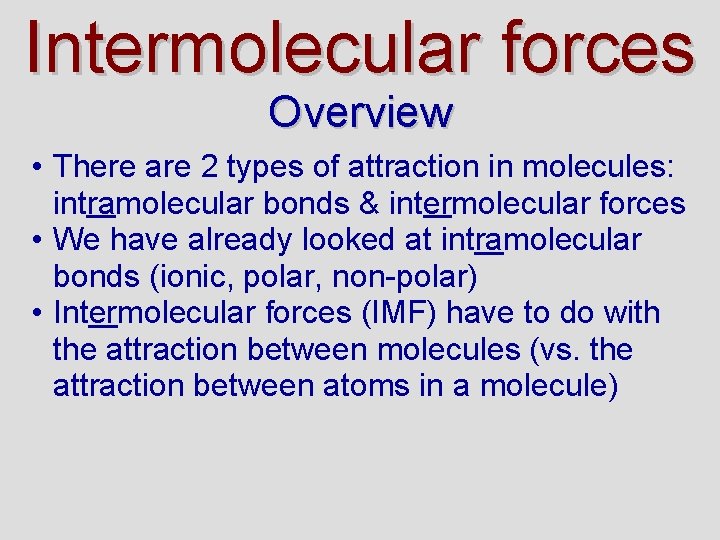
Ionic, Dipole - Dipole attractions • We have seen that molecules + – can have a separation of charge • This happens in both ionic and polar bonds (the greater the EN, the greater the dipoles) H Cl • Molecules are attracted to each other in a compound by these +σ and - σ forces + –

H - bonding • H-bonding is a special type of dipole - dipole attraction that is very strong • It occurs when N, O, or F are bonded to H Q- Calculate the EN for HCl and H 2 O A- HCl = 2. 9 -2. 1 = 0. 8, H 2 O = 3. 5 -2. 1 = 1. 4 • The high EN of NH, OH, and HF bonds cause these to be strong forces (about 5 x stronger than normal dipole-dipole forces) • They are given a special name (H-bonding) because compounds containing these bonds are important in biological systems

Hydrogen Bond • Strongest of all “weak” forces • Is caused when H is bonded to F, O, or N • These are so electronegative that the H is a “naked nucleus” or bare proton • Very attractive! • Will bond to nearby electron pairs

Importance of Hydrogen Bonding • Biological systems – DNA, proteins • Water chemistry (MP, BP, specific heat, surface tension) • Density of ice

Density • Most solids are more dense than liquid • Water is less dense because of hydrogen bonding • At 4°C, water becomes less dense • Important for life in winter • Causes lake turnover • Alum example

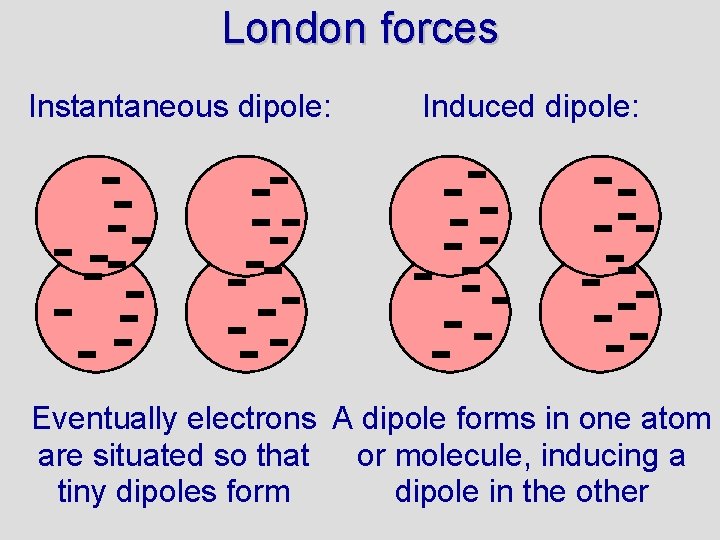
London forces • Non-polar molecules do not have dipoles like polar molecules. How, then, can non-polar compounds form solids or liquids? • London forces are named after Fritz London (also called van der Waal forces) • London forces are due to small dipoles that exist in non-polar molecules • Because electrons are moving around in atoms there will be instants when the charge around an atom is not symmetrical • The resulting tiny dipoles cause attractions between atoms/molecules

London forces Instantaneous dipole: Induced dipole: Eventually electrons A dipole forms in one atom are situated so that or molecule, inducing a tiny dipoles form dipole in the other

London Dispersion Forces • • All molecules have this Only attraction in nonpolar molecules How can Iodine be a solid? Temporary lopsided charge builds up from random motion of electrons - 1930 • Increases with mass – we say it has greater polarizability • Straight molecule is more polarizable than a curled up molecule – why? • Halogen Family is a great essay

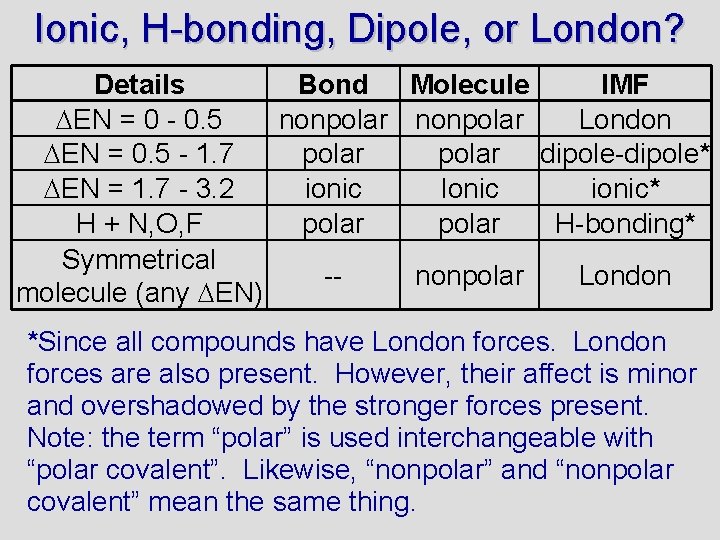
Ionic, H-bonding, Dipole, or London? Details Bond Molecule IMF EN = 0 - 0. 5 nonpolar London EN = 0. 5 - 1. 7 polar dipole-dipole* EN = 1. 7 - 3. 2 ionic Ionic ionic* H + N, O, F polar H-bonding* Symmetrical -nonpolar London molecule (any EN) *Since all compounds have London forces are also present. However, their affect is minor and overshadowed by the stronger forces present. Note: the term “polar” is used interchangeable with “polar covalent”. Likewise, “nonpolar” and “nonpolar covalent” mean the same thing.

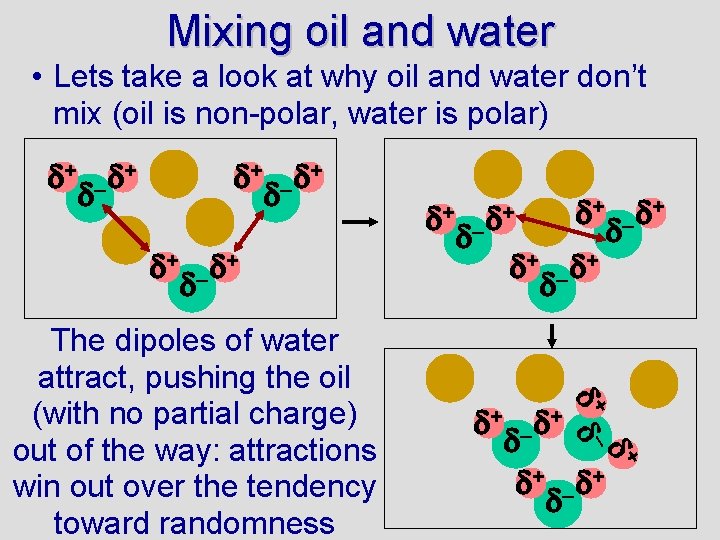
Mixing oil and water • Lets take a look at why oil and water don’t mix (oil is non-polar, water is polar) + – + + – + + + + – The dipoles of water attract, pushing the oil (with no partial charge) out of the way: attractions win out over the tendency toward randomness +

Properties of Liquids • • Viscosity “Slower than…. . Resistance of a liquid to flow Time it as it goes through a small tube with gravity acting upon it. • Poise – 1 g/cm-s • Trends – same substance – decreases with increasing temperature series (same structure) – increases with increasing mass

Surface Tension • • How many drops on a penny? Uneven forces at surface Acts like pond scum Definition – energy needed to increase the surface area of a liquid by a certain amount » Water is high – why? • • Called “cohesive” force – together Water moving up a stem – adhesive force Capillary acion – rise up a thin tube Meniscus!

Phase Changes • Solid to Liquid is called Heat of Fusion Hfus For water, 6 k. J/mol Liquid to Gas is called Heat of Vaporization Hvap For water, 40. 7 k. J/mol Hsub is sum of each

Heating Curve • Try a problem • Remember - flat during phase change, temperature change when heating a single phase • Cooling is opposite

Supercooling • Happens with some liquids - remove heat and it doesn’t freeze when it should • Very unstable • May happen during hibernation

Critical Temperature • Highest temperature at which a liquid can form from a gas when pressure is applied. • Above this, the substance is called a supercritical fluid. • Gas just becomes more compressed. • Critical pressure - pressure at the critical temperature

Vapor pressure • Vapor pressure forms above any liquid if container is closed – why? • Equilibrium is reached • This is vapor pressure • Higher if forces holding liquid together are weak - called a volatile (fleeing) liquid

Boiling Point • Temperature at which the VP equals atmospheric pressure • Normal BP - boiling point at 1 atm • Everest? Autoclave?

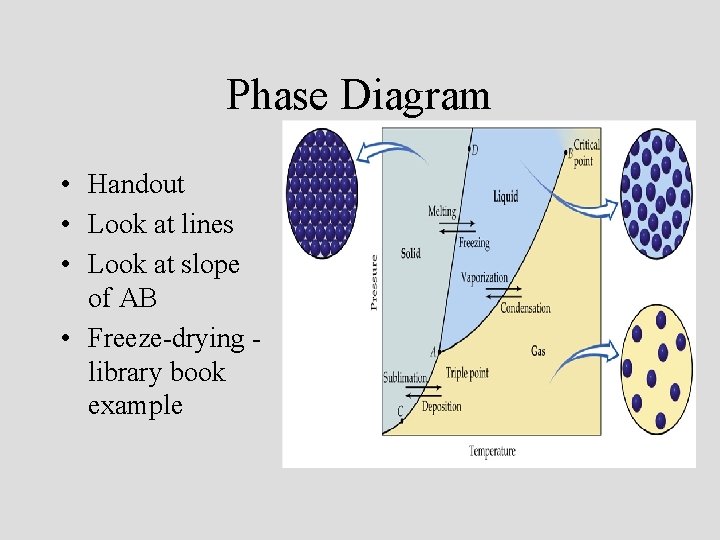
Phase Diagram • Handout • Look at lines • Look at slope of AB • Freeze-drying library book example

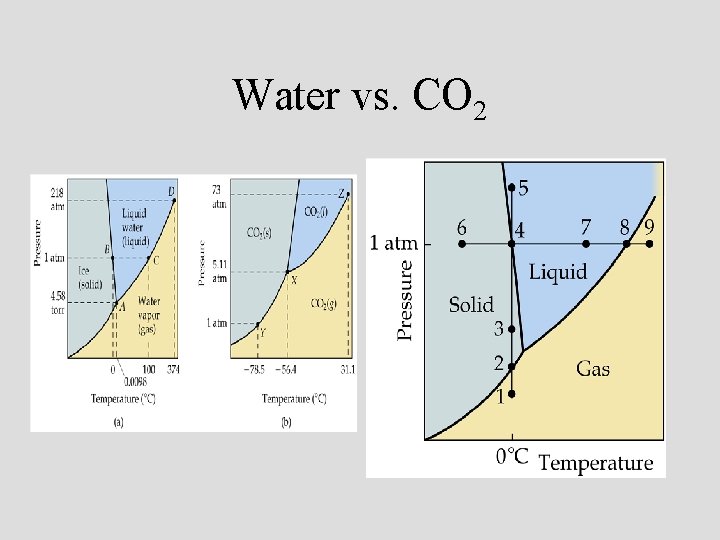
Water vs. CO 2

Structure of Solids • Amorphous (rubber, plastics) - large or mixtures - no true structure • Crystalline - highly ordered structure • Crystalline solids have true melting points

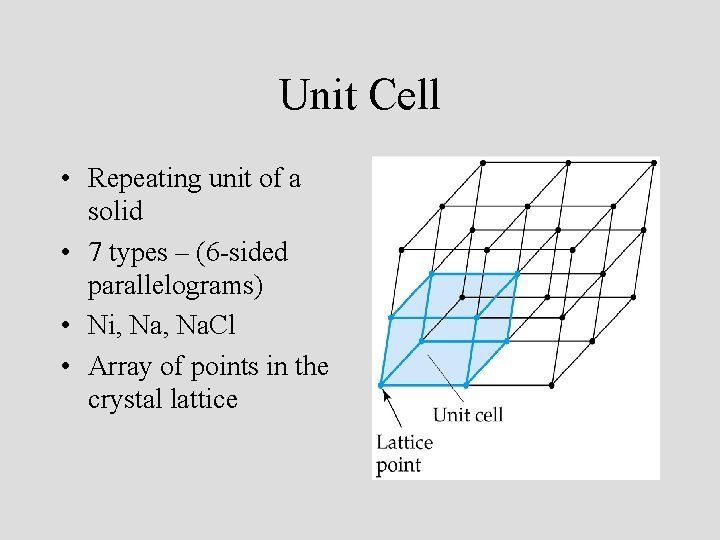
Unit Cell • Repeating unit of a solid • 7 types – (6 -sided parallelograms) • Ni, Na. Cl • Array of points in the crystal lattice

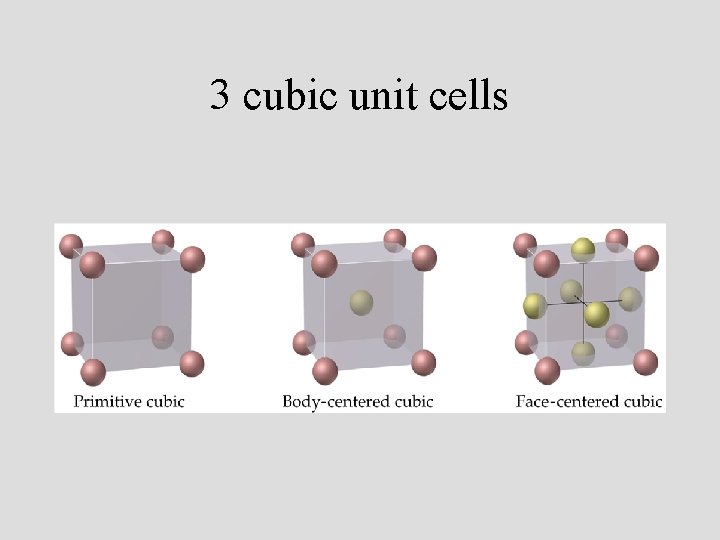
3 cubic unit cells

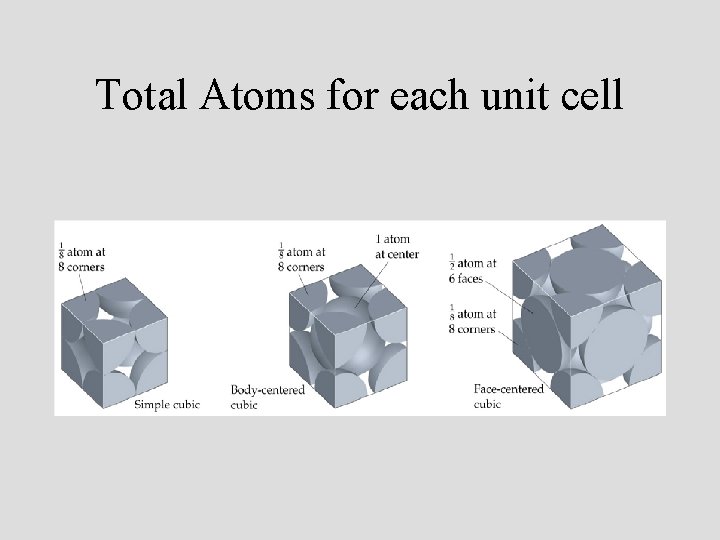
Total Atoms for each unit cell

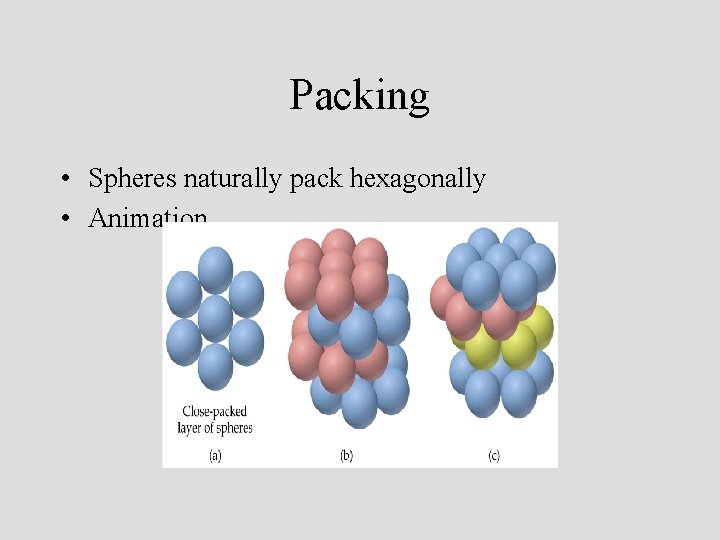
Packing • Spheres naturally pack hexagonally • Animation

Bonding • • Shown by x-ray diffraction Molecular - low MP If unit packs well, mp can be high Covalent Network Solid - very strong Many covalent bonds in 3 -D Diamond, graphite, Si. O 2, Si. C, BN Ionic - greater charge, greater MP Metallic solids - hexagonal close packed, mp varies

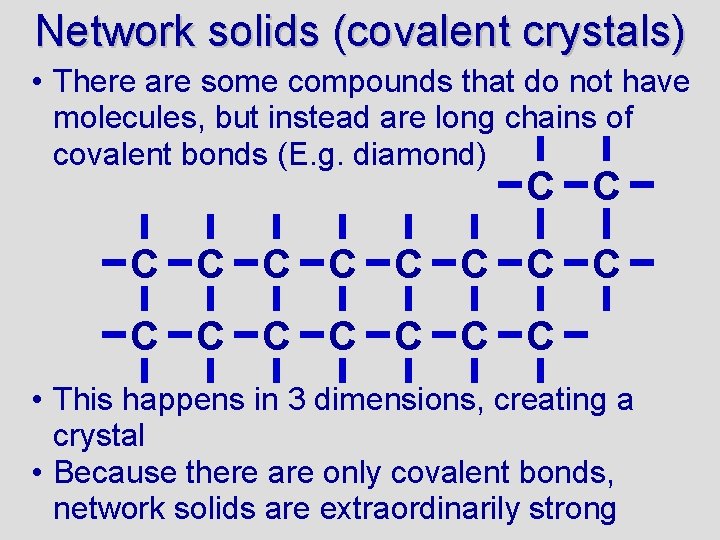
Network solids (covalent crystals) • There are some compounds that do not have molecules, but instead are long chains of covalent bonds (E. g. diamond) C C C C C • This happens in 3 dimensions, creating a crystal • Because there are only covalent bonds, network solids are extraordinarily strong

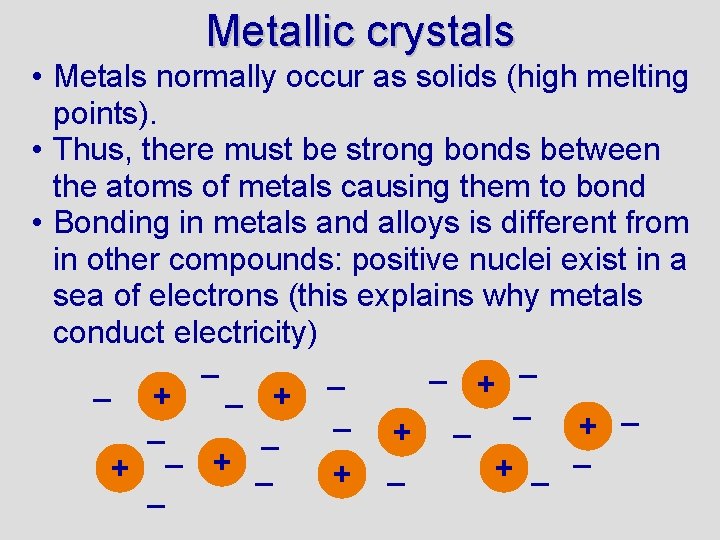
Metallic crystals • Metals normally occur as solids (high melting points). • Thus, there must be strong bonds between the atoms of metals causing them to bond • Bonding in metals and alloys is different from in other compounds: positive nuclei exist in a sea of electrons (this explains why metals conduct electricity) – – – + – + – + – –

Crystal types • There are 6 types of intermolecular forces • These forces are associated with certain crystal types. By comparing solids we have a common frame of reference. • The crystal types and their basic units are 1) Network (covalently bonded atoms) 2) ionic (electrostatic attraction of ions), 3) metallic (positive nuclei in electron sea), 4) Molecular (electrostatic attraction of dipoles in molecules) a) Polar (dipole-dipole and H-bonding) b) Non-polar (London forces)

Properties of crystals • Boiling and melting occur when the forces between molecules are overcome and a change of state occurs • The higher the force of attraction between molecules (IMF) the higher the melting/boiling point (see previous slide for order) • Only metallic crystals conduct electricity in solid state (they also conduct in liquid state) • Ionic crystals will conduct electricity in molten state or dissolved because ions are free to move to positive and negative poles

Solubility of crystal types • Solute = what is dissolving (e. g. salt) • Solvent = what it is dissolving in (e. g. water) • Strong attractions between the basic units of covalent crystals cause them to be insoluble. • Metallic crystals are likewise insoluble • The solubility of other crystals depends on solute and solvent characteristics • We will see that polar/ionic solutes dissolve in polar/ionic solvents and non-polar solutes dissolve in non-polar solvents • This is known as the like-dissolves-like rule

Crystals worksheet • Place numbers in boxes according to descriptions • Use pg. 431 -439 and class notes, and internet as references • At end rows should add up to 126 each

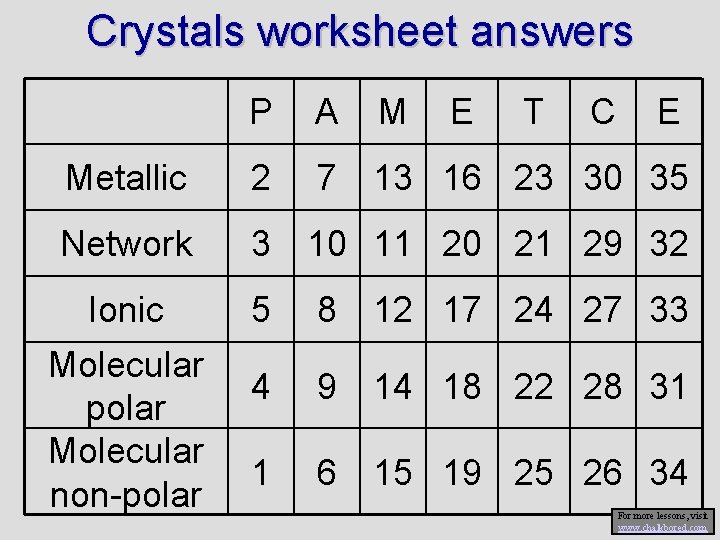
Crystals worksheet answers P A M Metallic 2 7 13 16 23 30 35 Network 3 10 11 20 21 29 32 Ionic Molecular polar Molecular non-polar E T C E 5 8 12 17 24 27 33 4 9 14 18 22 28 31 1 6 15 19 25 26 34 For more lessons, visit www. chalkbored. com

Testing concepts 1. Which attractions are stronger: intermolecular or intramolecular? 2. How many times stronger is a covalent bond compared to a dipole-dipole attraction? 3. What evidence is there that nonpolar molecules attract each other? 4. Suggest some ways that the dipoles in London forces are different from the dipoles in dipole-dipole attractions. 5. A) Which would have a lower boiling point: O 2 or F 2? Explain. B) Which would have a lower boiling point: NO or O 2? Explain.

7. Which would you expect to have the higher melting point (or boiling point): C 8 H 18 or C 4 H 10? Explain. 8. What two factors causes hydrogen bonds to be so much stronger than typical dipole-dipole bonds? 9. So far we have discussed 4 kinds of intermolecular forces: ionic, dipole-dipole, hydrogen bonding, and London forces. What kind(s) of intermolecular forces are present in the following substances: a) NH 3, b) SF 6, c) PCl 3, d) Li. Cl, e) HBr, f) CO 2 (hint: consider EN and molecular shape/polarity) Challenge: Ethanol (CH 3 CH 2 OH) and dimethyl ether (CH 3 OCH 3) have the same formula (C 2 H 6 O). Ethanol boils at 78 C, whereas dimethyl ether boils at -24 C. Explain why the boiling point of the ether is so much lower than the boiling point of ethanol. Challenge: try answering the question on the next slide.

Testing concepts 1. Intramolecular are stronger. 2. A covalent bond is 100 x stronger. 3. The molecules gather together as liquids or solids at low temperatures. 4. London forces – Are present in all compounds – Can occur between atoms or molecules – Are due to electron movement not to EN – Are transient in nature (dipole-dipole are more permanent). – London forces are weaker

Testing concepts 6. A) F 2 would be lower because it is smaller. Larger atoms/molecules can have their electron clouds more easily deformed and thus have stronger London attractions and higher melting/boiling points. B) O 2 because it has only London forces. NO has a small EN, giving it small dipoles. 7. C 8 H 18 would have the higher melting/boiling point. This is a result of the many more sites available for London forces to form. 8. 1) a large EN, 2) the small sizes of atoms.

Testing concepts 9. a) NH 3: Hydrogen bonding (H + N), London. b) SF 6: London only (it is symmetrical). c) PCl 3: EN=2. 9 -2. 1. Dipole-dipole, London. d) Li. Cl: EN=2. 9 -1. 0. Ionic, (London). e) HBr: EN=2. 8 -2. 1. Dipole-dipole, London. f) CO 2: London only (it is symmetrical) Challenge: In ethanol, H and O are bonded (the large EN results in H-bonding). In dimethyl ether the O is bonded to C (a smaller EN results in a dipole-dipole attraction rather than hydrogen bonding).

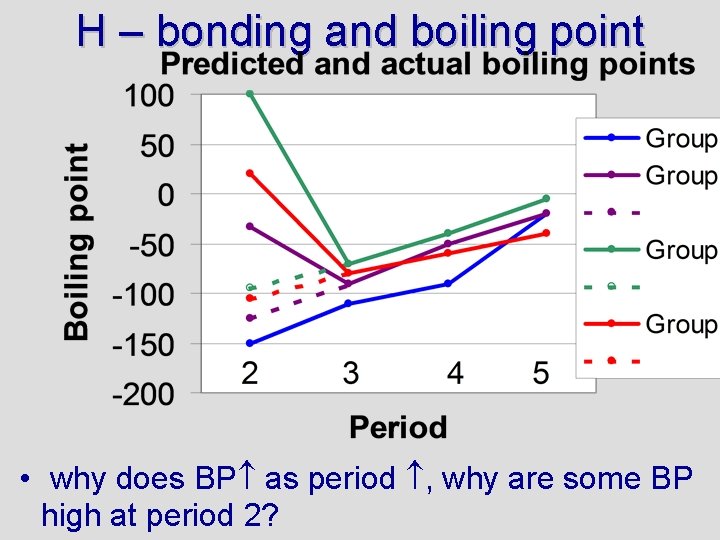
H – bonding and boiling point • why does BP as period , why are some BP high at period 2?

Testing concepts Boiling points increase down a group (as period increases) for two reasons: 1) EN tends to increase and 2) size increases. A larger size means greater London forces. Boiling points are very high for H 2 O, HF, and NH 3 because these are hydrogen bonds (high EN), creating large intermolecular forces For more lessons, visit www. chalkbored. com