Quantum Regime At the atomic scale classical physics






- Slides: 33

Quantum “Regime” • At the atomic scale, classical physics ideas aren’t sufficient. All particles have wavelike nature, all waves are at the same time composed of particles. • Usually this is not apparent, as we shall see – large scale or heavy objects tend to have such very short wavelengths that they appear “crisp”, as we normally experience • The word “quantum” arose originally when Max Planck deduced that to explain the spectrum of wavelengths of EM radiation emitted by a black body at various temperatures, it was necessary to postulate that EM energy is QUANTIZED in little packages of size directly proportional to their frequency. Introduction

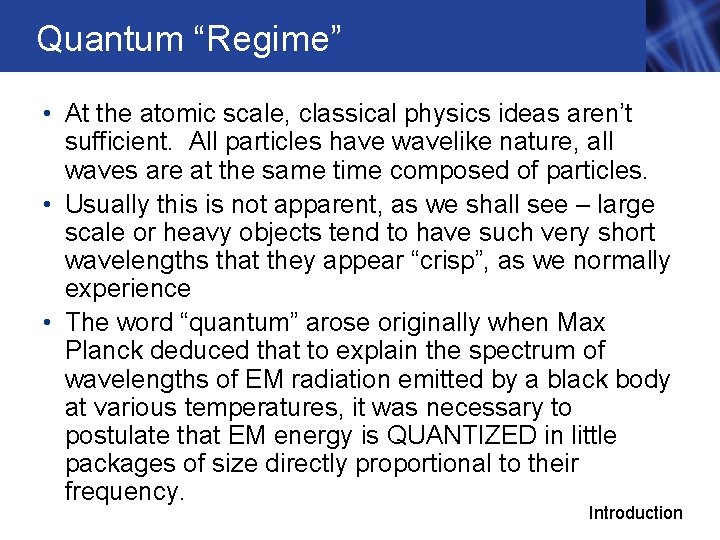
Waves vs. Particles • Waves produce an interference pattern when passed through a double slit • Classical particles (bullets) can pass through only one of the slits, and no interference pattern will be formed Section 28. 1

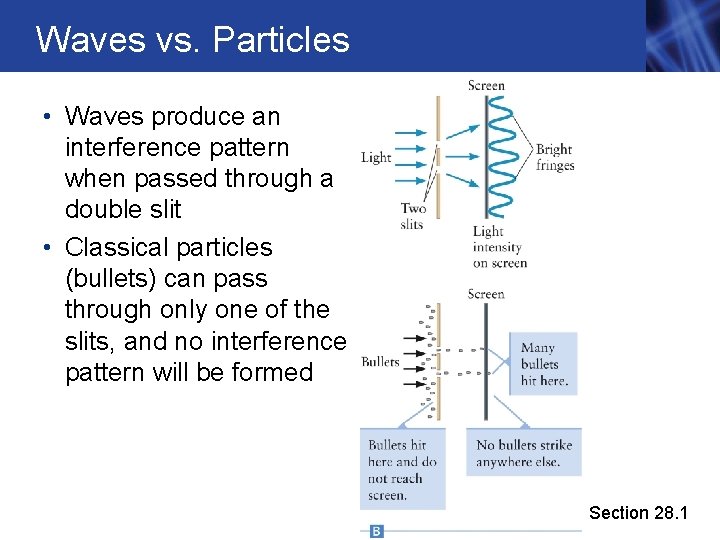
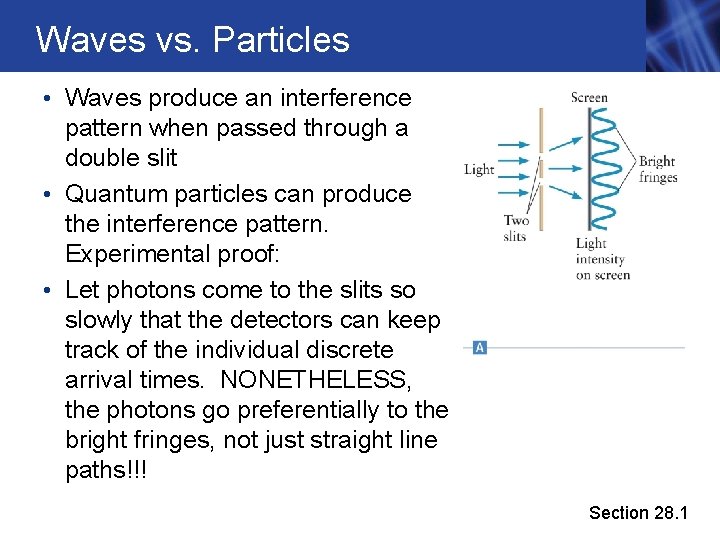
Waves vs. Particles • Waves produce an interference pattern when passed through a double slit • Quantum particles can produce the interference pattern. Experimental proof: • Let photons come to the slits so slowly that the detectors can keep track of the individual discrete arrival times. NONETHELESS, the photons go preferentially to the bright fringes, not just straight line paths!!! Section 28. 1

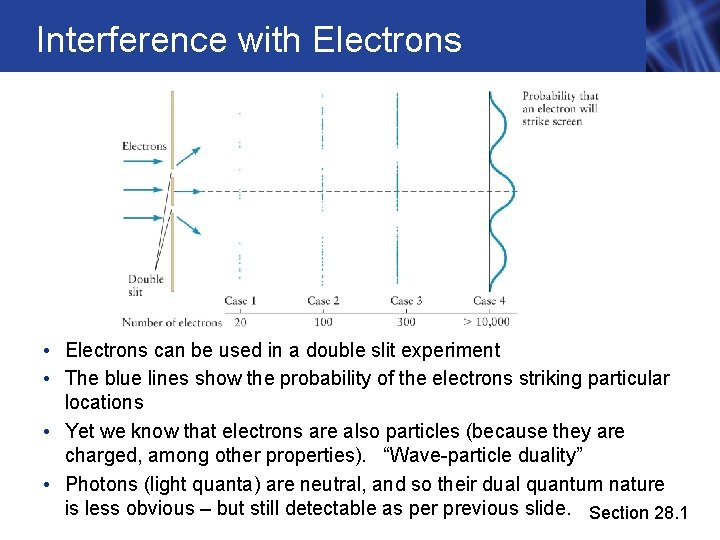
Interference with Electrons • Electrons can be used in a double slit experiment • The blue lines show the probability of the electrons striking particular locations • Yet we know that electrons are also particles (because they are charged, among other properties). “Wave-particle duality” • Photons (light quanta) are neutral, and so their dual quantum nature is less obvious – but still detectable as per previous slide. Section 28. 1

Interference with Electrons, cont. • The probability pattern of the electrons has the same form as the variation of light intensity in the double-slit interference experiment • The experiment shows that electrons undergo constructive and destructive interference at certain locations on the screen • The experiment also shows aspects of particle-like behavior since the electrons arrive one at a time at the screen, and also don’t just go in straight lines. • In principle, you can slowly build up an interference pattern over time even if only one electron (or photon) per second arrives at the screen, for example. Weird! Section 28. 1

Work Function • Already in the 1880 s, studies of what happens when light is shone onto a metal gave some results that could not be explained with the wave theory of light • The work function, Wc is the minimum energy required to remove a single electron from a piece of metal Section 28. 2

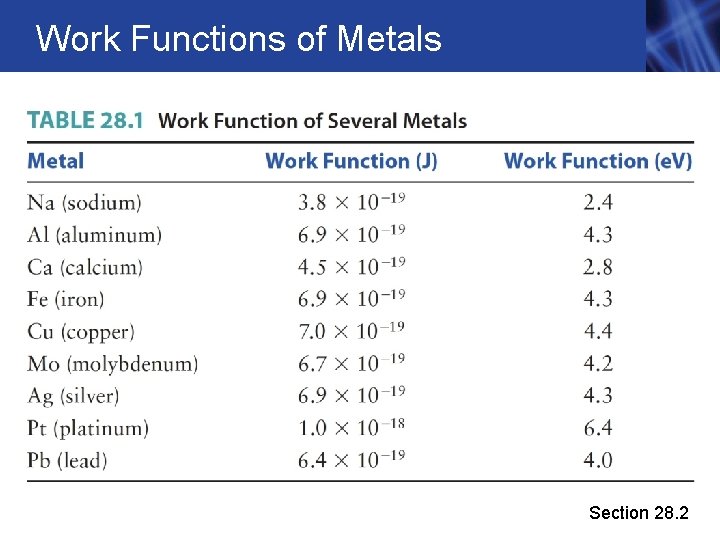
Work Functions of Metals Section 28. 2

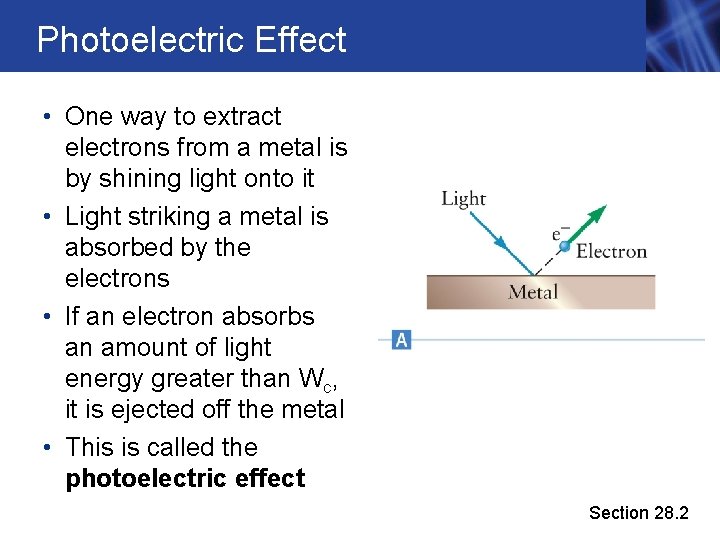
Photoelectric Effect • One way to extract electrons from a metal is by shining light onto it • Light striking a metal is absorbed by the electrons • If an electron absorbs an amount of light energy greater than Wc, it is ejected off the metal • This is called the photoelectric effect Section 28. 2

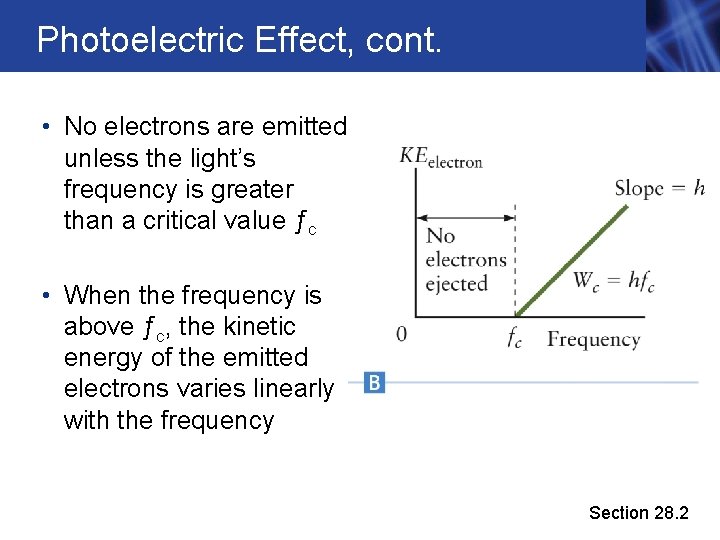
Photoelectric Effect, cont. • No electrons are emitted unless the light’s frequency is greater than a critical value ƒc • When the frequency is above ƒc, the kinetic energy of the emitted electrons varies linearly with the frequency Section 28. 2

Photoelectric Effect, Explanation • Trying to explain the photoelectric effect with the classical wave theory of light presented two difficulties • Experiments showed that the critical frequency is independent of the intensity of the light • Remember, the E field in a light wave goes as the square root of the intensity. You’d think a big enough E field would manage to eject electrons. Wrong! • Below the critical frequency, there are no ejected electrons no matter how great the light intensity • Also the KE of ejected electrons depends on the light frequency, NOT intensity. Both phenomena lead to the same conclusion. Section 28. 2

Photons • Einstein proposed that light carries energy in discrete quanta, now called photons • Each photon carries a parcel of energy Ephoton = hƒ • h is a constant of nature called Planck’s constant • h = 6. 626 x 10 -34 J ∙ s TINY, measures the scale of quantum phenomena • A beam of light should be thought of as a collection of photons (as well as a wave, which it also is!) • Each photon has an energy dependent on its frequency • If the intensity of monochromatic light is increased, the number of photons is increased, but the energy carried by each photon does not change Section 28. 2

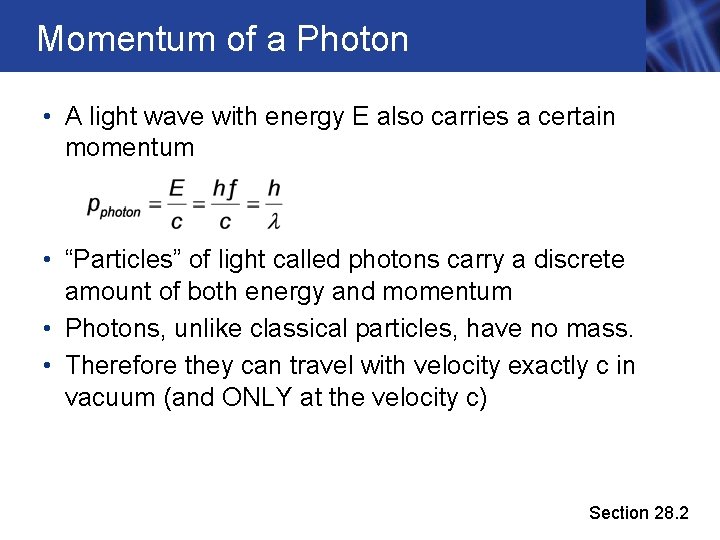
Momentum of a Photon • A light wave with energy E also carries a certain momentum • “Particles” of light called photons carry a discrete amount of both energy and momentum • Photons, unlike classical particles, have no mass. • Therefore they can travel with velocity exactly c in vacuum (and ONLY at the velocity c) Section 28. 2

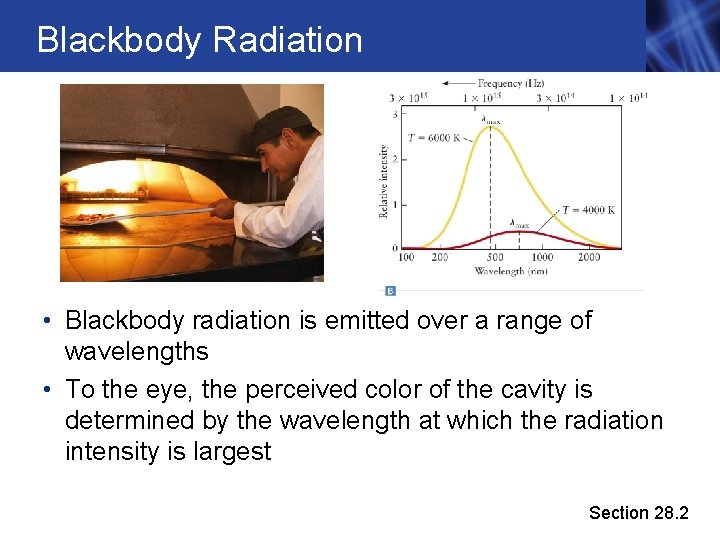
Blackbody Radiation • Blackbody radiation is emitted over a range of wavelengths • To the eye, the perceived color of the cavity is determined by the wavelength at which the radiation intensity is largest Section 28. 2

Blackbody Radiation, Classical • The blackbody intensity curve has the same shape for a wide variety of objects • Electromagnetic waves can form standing waves as they reflect back and forth inside the oven’s cavity • The frequencies of the standing waves follow the pattern ƒn = n ƒ where n = 1, 2, 3, … • There is no limit to the value of n, so the frequency can be infinitely large • But as the frequency increases, so does the energy • Classical theory predicts that the blackbody intensity should become infinite as the frequency approaches infinity Section 28. 2

Blackbody Radiation, Quantum • The disagreement between the classical predictions and experimental observations was called the “ultraviolet catastrophe” • Planck proposed solving the problem by assuming the energy in a blackbody cavity must come in discrete parcels • Each parcel would have energy E = h ƒn • His theory (formula) fit the experimental results, but gave no theoretical reason why the formula worked • Planck’s work is generally considered to be the beginning of quantum theory. • Einstein gave theoretical underpinning in 1905. Section 28. 2

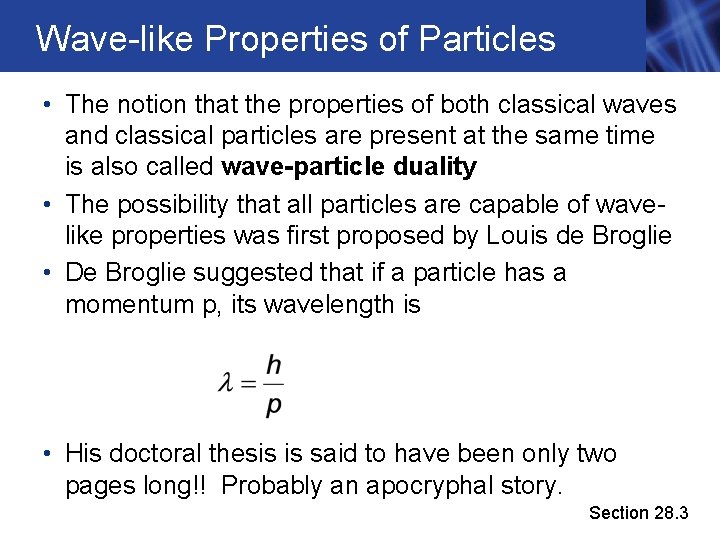
Wave-like Properties of Particles • The notion that the properties of both classical waves and classical particles are present at the same time is also called wave-particle duality • The possibility that all particles are capable of wavelike properties was first proposed by Louis de Broglie • De Broglie suggested that if a particle has a momentum p, its wavelength is • His doctoral thesis is said to have been only two pages long!! Probably an apocryphal story. Section 28. 3

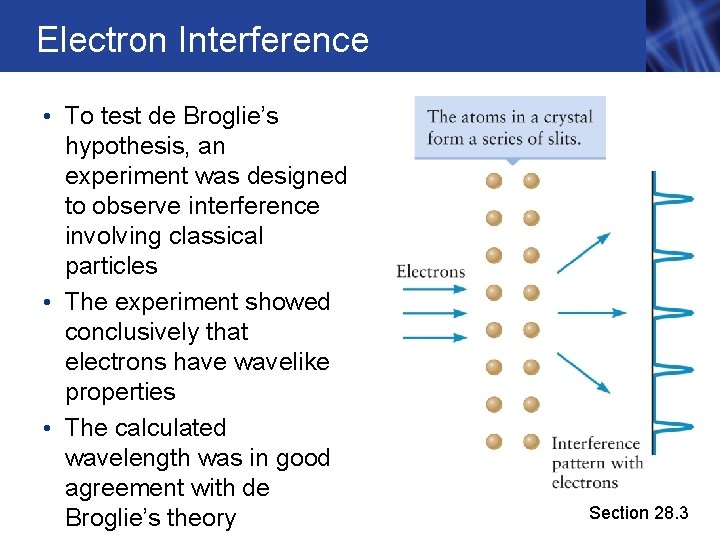
Electron Interference • To test de Broglie’s hypothesis, an experiment was designed to observe interference involving classical particles • The experiment showed conclusively that electrons have wavelike properties • The calculated wavelength was in good agreement with de Broglie’s theory Section 28. 3

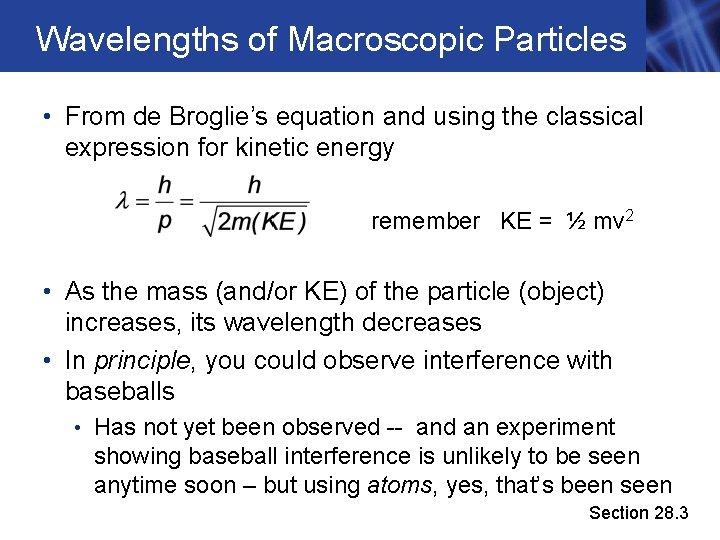
Wavelengths of Macroscopic Particles • From de Broglie’s equation and using the classical expression for kinetic energy remember KE = ½ mv 2 • As the mass (and/or KE) of the particle (object) increases, its wavelength decreases • In principle, you could observe interference with baseballs • Has not yet been observed -- and an experiment showing baseball interference is unlikely to be seen anytime soon – but using atoms, yes, that’s been seen Section 28. 3

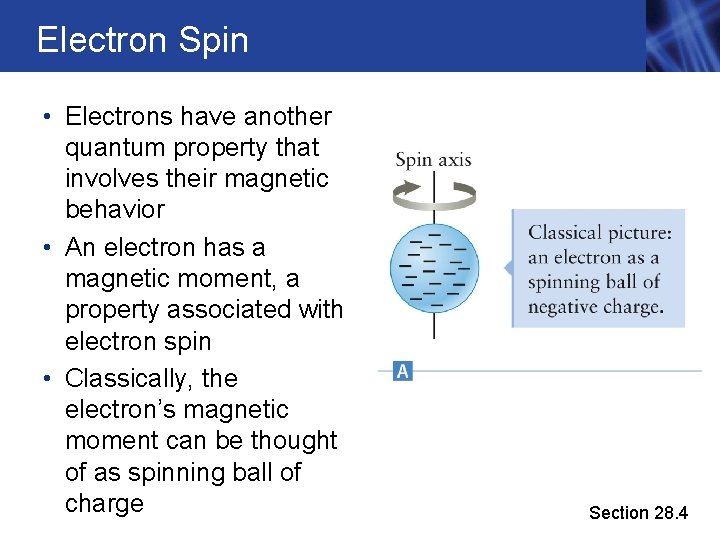
Electron Spin • Electrons have another quantum property that involves their magnetic behavior • An electron has a magnetic moment, a property associated with electron spin • Classically, the electron’s magnetic moment can be thought of as spinning ball of charge Section 28. 4

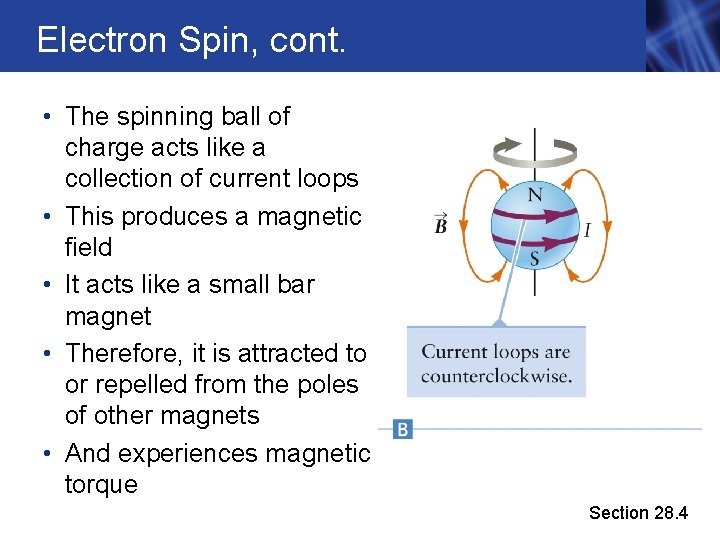
Electron Spin, cont. • The spinning ball of charge acts like a collection of current loops • This produces a magnetic field • It acts like a small bar magnet • Therefore, it is attracted to or repelled from the poles of other magnets • And experiences magnetic torque Section 28. 4

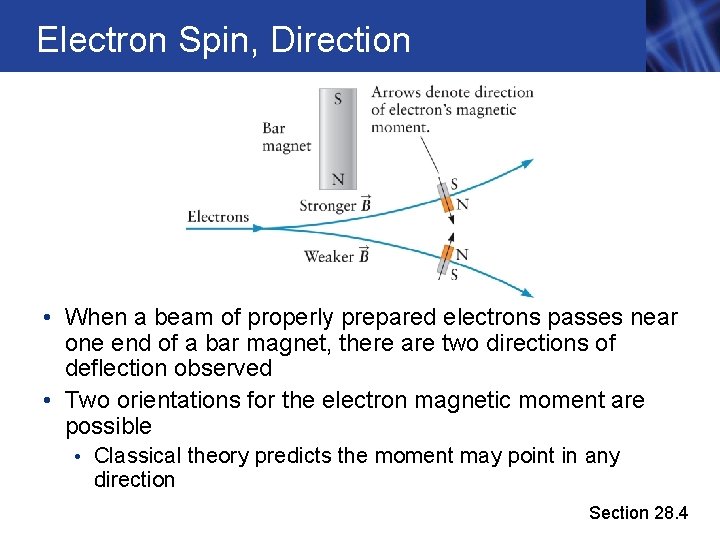
Electron Spin, Direction • When a beam of properly prepared electrons passes near one end of a bar magnet, there are two directions of deflection observed • Two orientations for the electron magnetic moment are possible • Classical theory predicts the moment may point in any direction Section 28. 4

Electron Spin, Direction, cont. • Classically, the electrons should deflect over a range of angles • Observing only two directions of deflection indicates there are only two possible orientations for the magnetic moment • The electron magnetic moment is quantized in direction with only two possible values • The magnitude of the electron’s magnetic moment is also quantized • Pick any axis in 3 -D. The spin of the electron is +the standard amount along the chosen direction. Super weird, but that’s the way it works Section 28. 4

Wave Function • In the quantum world, the motion of a particle-wave is described by its wave function • The wave function can be calculated from Schrödinger’s equation • Developed by Erwin Schrödinger, one of the inventors of quantum theory • Schrödinger’s equation plays a role similar to Newton’s laws of motion: it tells how the wave function evolves with time, just as Newton’s laws tell classically how a particle’s position, momentum, and energy evolve with time. • In many situations, the solutions of the Schrödinger equation are mathematically similar to standing waves Section 28. 5

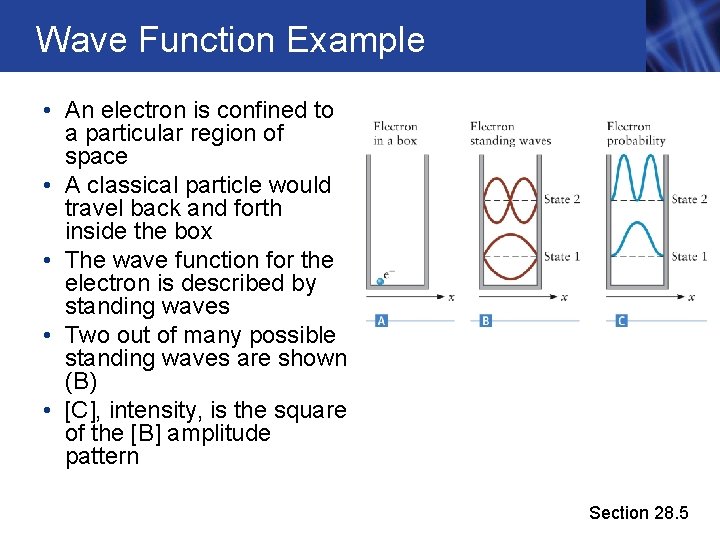
Wave Function Example • An electron is confined to a particular region of space • A classical particle would travel back and forth inside the box • The wave function for the electron is described by standing waves • Two out of many possible standing waves are shown (B) • [C], intensity, is the square of the [B] amplitude pattern Section 28. 5

Wave Function Example, cont. • The wave function solutions correspond to electrons with different kinetic energies • The wavelengths of the standing waves are different • Given by de Broglie’s equation • After finding the wave function, one can calculate the position and velocity of the electron • But does not give a single value • The wave function allows for the calculation of the probability of finding the electron at different locations in space • That’s how sparse electrons can slowly build up a wave interference pattern on a screen – each electron has a probability of arriving different places on the screen: on average more arrive in the “bright” directions, etc. Section 28. 5

Heisenberg Uncertainty Principle • Wavy quantum effects place fundamental limits on the precision of measuring position or velocity • Standing waves in the box are the electron, so there is an inherent uncertainty in its position • There is some probability for finding the electron at virtually any spot in the box • The uncertainty in position, Δx, is approximately the size of the box. Smaller box: smaller Δx and shorter wavelengths. Meaning higher frequencies. • Guess what has happened to the energy levels! Section 28. 5

Quiz • The uncertainty in position, Δx, is approximately the size of the box. Smaller box: smaller Δx and shorter wavelengths. Meaning higher frequencies. • Compared to a larger box, the energy levels in the smaller box are: • • A) B) C) D) the same smaller larger not determinable Section 28. 5

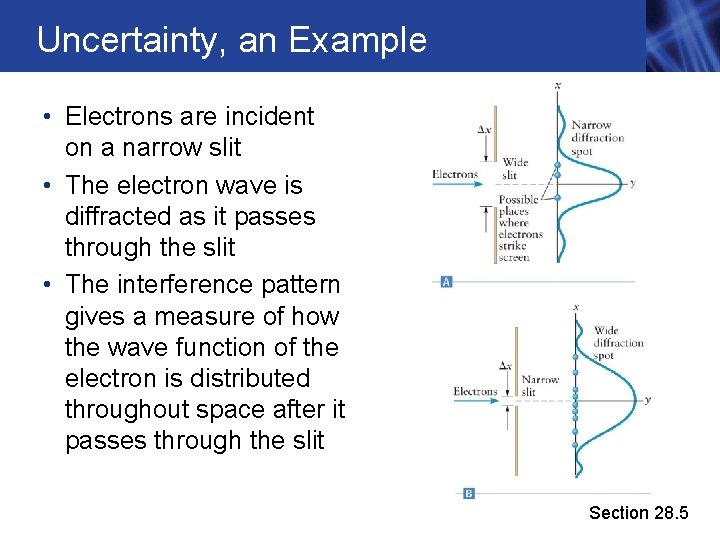
Uncertainty, an Example • Electrons are incident on a narrow slit • The electron wave is diffracted as it passes through the slit • The interference pattern gives a measure of how the wave function of the electron is distributed throughout space after it passes through the slit Section 28. 5

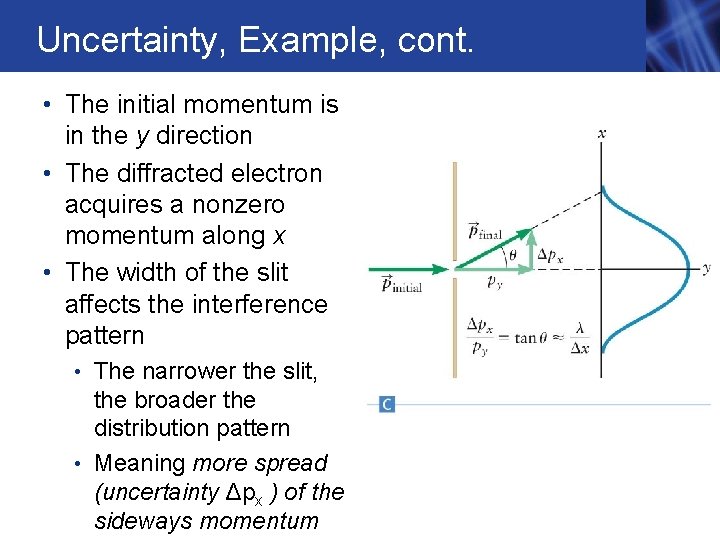
Uncertainty, Example, cont. • The initial momentum is in the y direction • The diffracted electron acquires a nonzero momentum along x • The width of the slit affects the interference pattern • The narrower the slit, the broader the distribution pattern • Meaning more spread (uncertainty Δpx ) of the sideways momentum

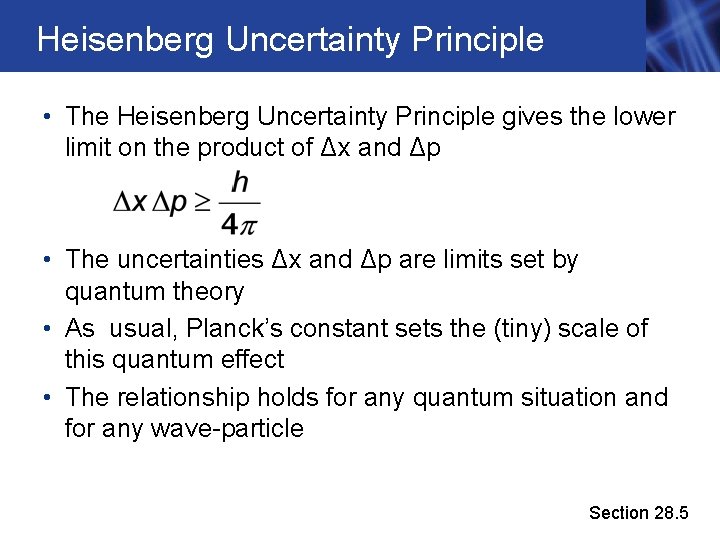
Heisenberg Uncertainty Principle • The Heisenberg Uncertainty Principle gives the lower limit on the product of Δx and Δp • The uncertainties Δx and Δp are limits set by quantum theory • As usual, Planck’s constant sets the (tiny) scale of this quantum effect • The relationship holds for any quantum situation and for any wave-particle Section 28. 5

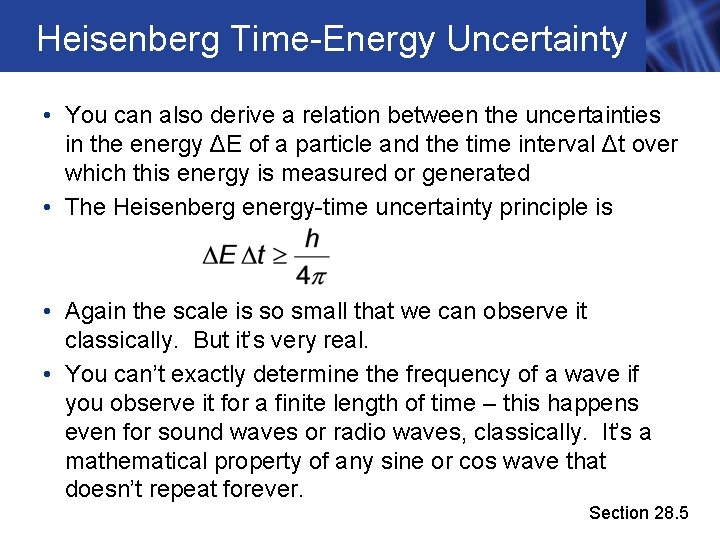
Heisenberg Time-Energy Uncertainty • You can also derive a relation between the uncertainties in the energy ΔE of a particle and the time interval Δt over which this energy is measured or generated • The Heisenberg energy-time uncertainty principle is • Again the scale is so small that we can observe it classically. But it’s very real. • You can’t exactly determine the frequency of a wave if you observe it for a finite length of time – this happens even for sound waves or radio waves, classically. It’s a mathematical property of any sine or cos wave that doesn’t repeat forever. Section 28. 5

Heisenberg Uncertainty Principle, final • Quantum theory and the uncertainty principle mean that there is always a trade-off between the uncertainties • It is not possible, even in principle, to have perfect knowledge of both x and px • This suggests that there is always some inherent uncertainty in our knowledge of the physical universe • Quantum theory says that the world is inherently unpredictable • For any macroscale object, the uncertainties in the actual measurement will always be much larger than the inherent uncertainties due to the Heisenberg uncertainty relation Section 28. 5

Heisenberg Uncertainty and Relativity • Relativity deals with 3 -D space and one time dimension x, y, z, ct we call this set a fourvector • Similarly, px, py, pz and E/c form a four-vector • So it’s entirely natural to have FOUR Heisenberg uncertainty relations, one each for the x, y, and z components, and one for the time component • There a lot of beautiful symmetries in nature that guide our construction of theories of the universe Section 28. 5