Quantum Physics Part 1 AP Physics J Ruffolo

- Slides: 13

Quantum Physics Part 1 AP Physics – J. Ruffolo, Ph. D.

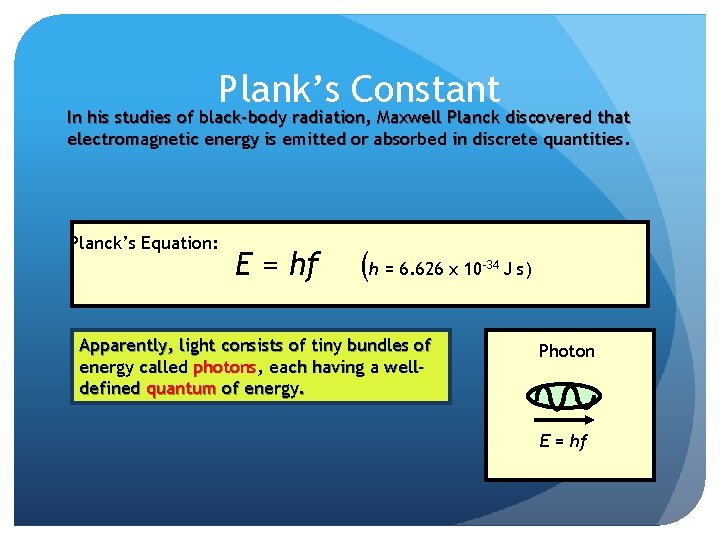
Plank’s Constant In his studies of black-body radiation, Maxwell Planck discovered that electromagnetic energy is emitted or absorbed in discrete quantities. Planck’s Equation: E = hf (h = 6. 626 x 10 Apparently, light consists of tiny bundles of energy called photons, each having a welldefined quantum of energy. -34 J s) Photon E = hf

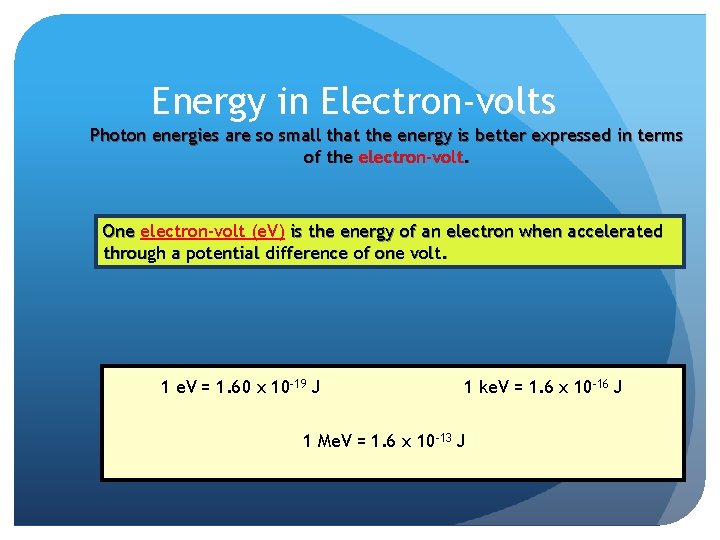
Energy in Electron-volts Photon energies are so small that the energy is better expressed in terms of the electron-volt. One electron-volt (e. V) is the energy of an electron when accelerated through a potential difference of one volt. 1 e. V = 1. 60 x 10 -19 J 1 ke. V = 1. 6 x 10 -16 J 1 Me. V = 1. 6 x 10 -13 J

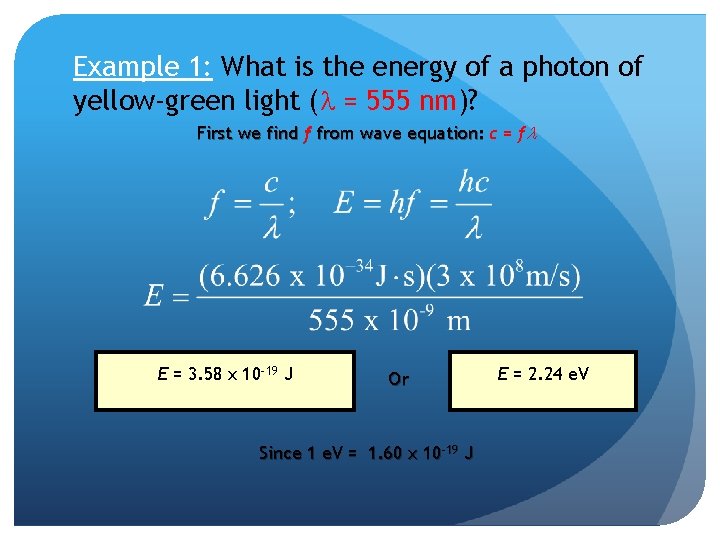
Example 1: What is the energy of a photon of yellow-green light (l = 555 nm)? First we find f from wave equation: c = fl E = 3. 58 x 10 -19 J Or Since 1 e. V = 1. 60 x 10 -19 J E = 2. 24 e. V

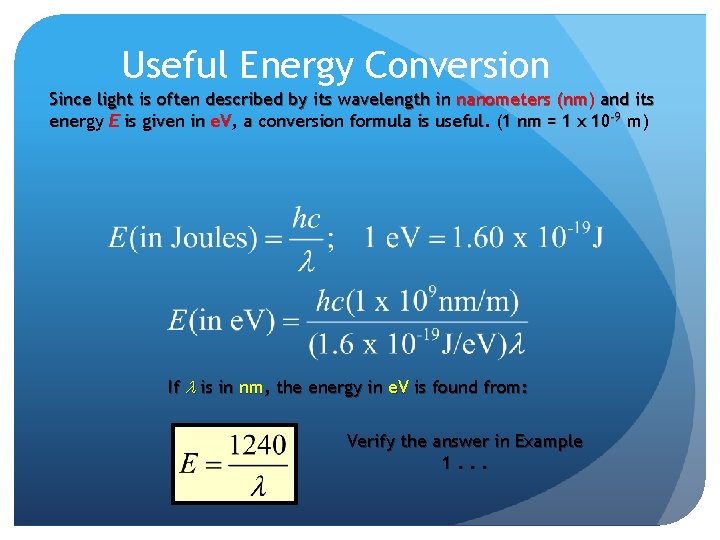
Useful Energy Conversion Since light is often described by its wavelength in nanometers (nm) and its energy E is given in e. V, a conversion formula is useful. (1 nm = 1 x 10 -9 m) If l is in nm, the energy in e. V is found from: Verify the answer in Example 1. . .

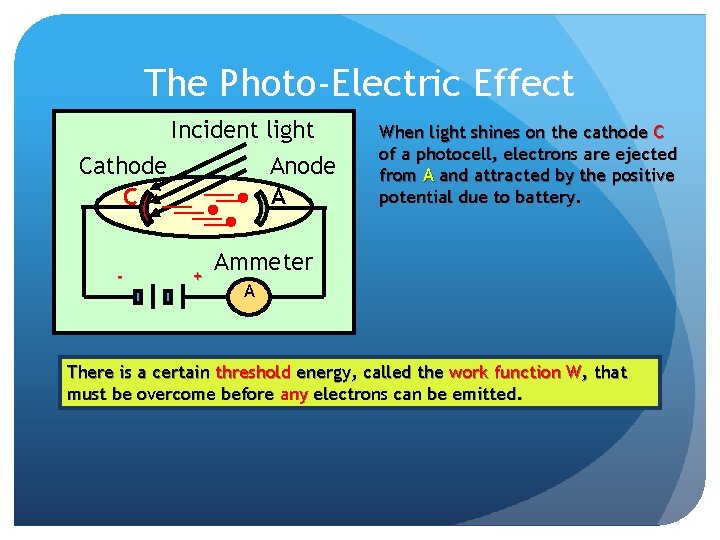
The Photo-Electric Effect Incident light Cathode C - Anode A + When light shines on the cathode C of a photocell, electrons are ejected from A and attracted by the positive potential due to battery. Ammeter A There is a certain threshold energy, called the work function W, that must be overcome before any electrons can be emitted.

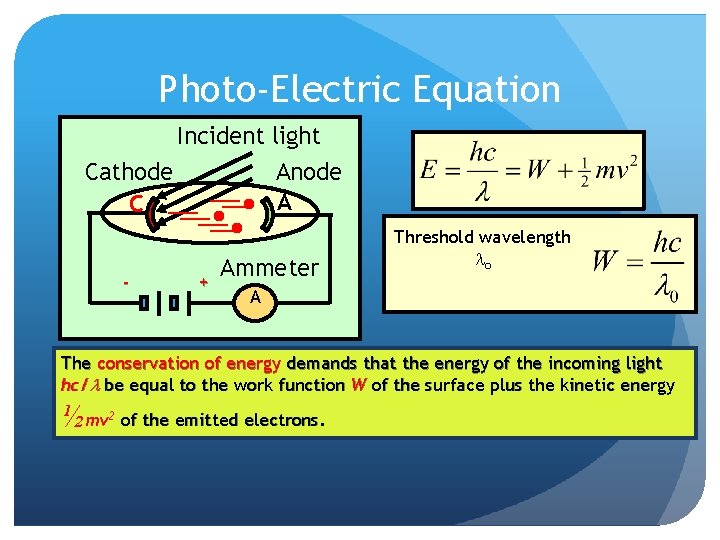
Photo-Electric Equation Incident light Cathode C - Anode A + Ammeter Threshold wavelength lo A The conservation of energy demands that the energy of the incoming light hc/l be equal to the work function W of the surface plus the kinetic energy ½mv 2 of the emitted electrons.

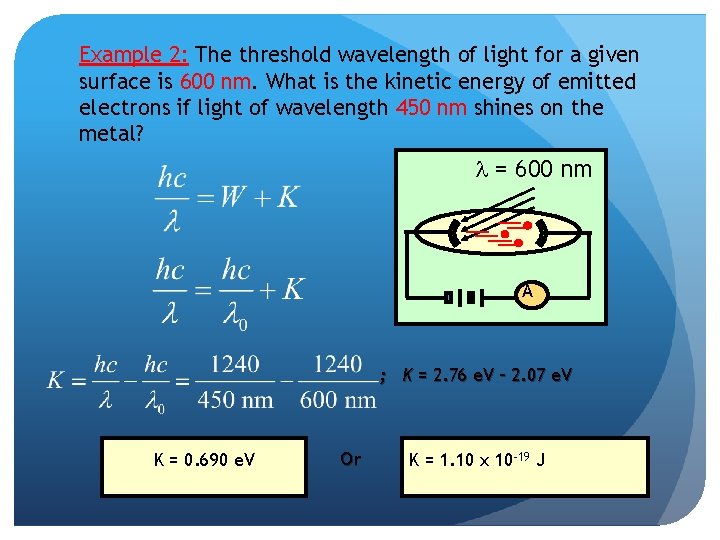
Example 2: The threshold wavelength of light for a given surface is 600 nm. What is the kinetic energy of emitted electrons if light of wavelength 450 nm shines on the metal? l = 600 nm A ; K = 2. 76 e. V – 2. 07 e. V K = 0. 690 e. V Or K = 1. 10 x 10 -19 J

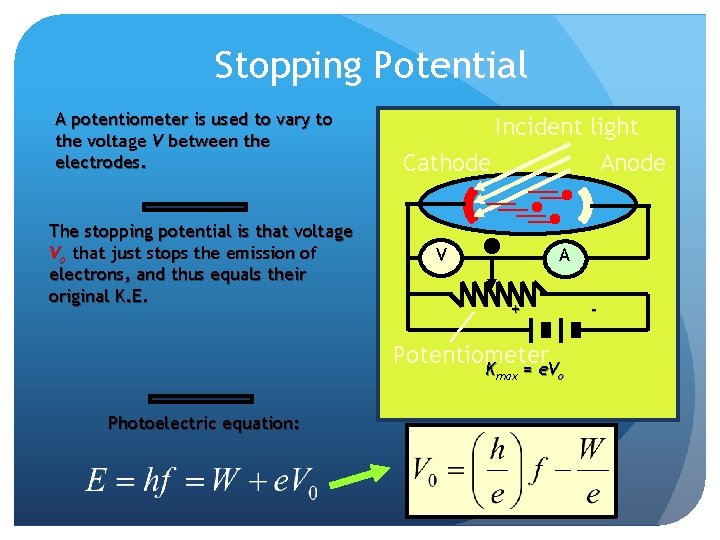
Stopping Potential A potentiometer is used to vary to the voltage V between the electrodes. The stopping potential is that voltage Vo that just stops the emission of electrons, and thus equals their original K. E. Incident light Cathode Anode V A + Potentiometer Kmax = e. Vo Photoelectric equation: -

Slope of a Straight Line (Review) The general equation for a straight line is: The slope of a line: y y = mx + b The x-intercept xo occurs when line crosses x axis or when y = 0. The slope of the line is the rise over the run: Slope y x xo x

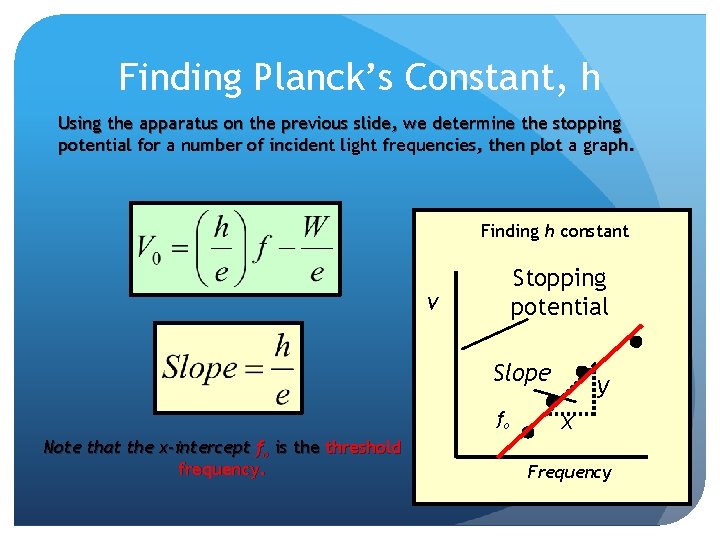
Finding Planck’s Constant, h Using the apparatus on the previous slide, we determine the stopping potential for a number of incident light frequencies, then plot a graph. Finding h constant Stopping potential V Slope fo Note that the x-intercept fo is the threshold frequency. y x Frequency

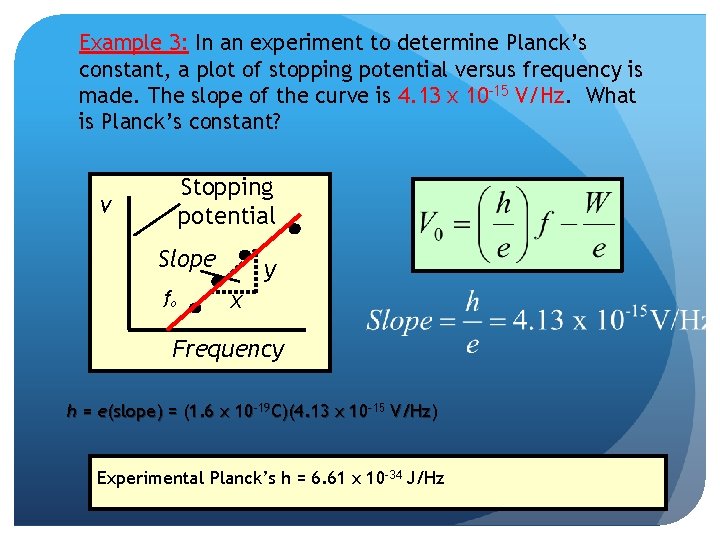
Example 3: In an experiment to determine Planck’s constant, a plot of stopping potential versus frequency is made. The slope of the curve is 4. 13 x 10 -15 V/Hz. What is Planck’s constant? Stopping potential V Slope fo y x Frequency h = e(slope) = (1. 6 x 10 -19 C)(4. 13 x 10 -15 V/Hz) Experimental Planck’s h = 6. 61 x 10 -34 J/Hz

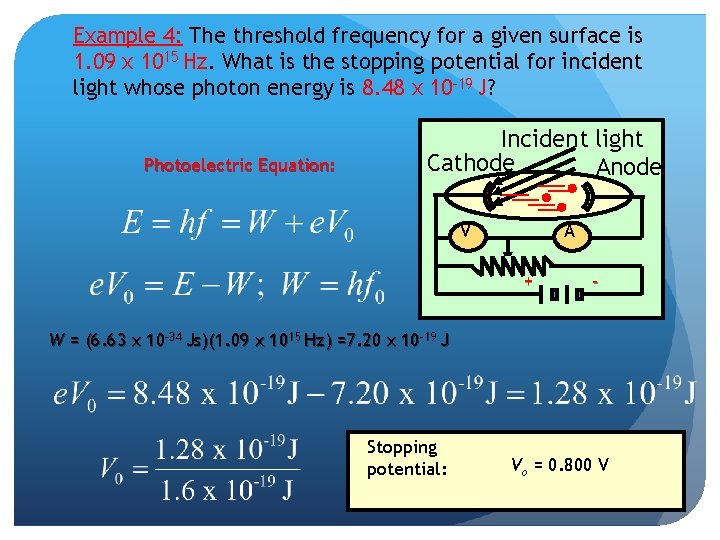
Example 4: The threshold frequency for a given surface is 1. 09 x 1015 Hz. What is the stopping potential for incident light whose photon energy is 8. 48 x 10 -19 J? Photoelectric Equation: Incident light Cathode Anode V A + - W = (6. 63 x 10 -34 Js)(1. 09 x 1015 Hz) =7. 20 x 10 -19 J Stopping potential: Vo = 0. 800 V