Quantum Physics Module Summary Elliott Waves or particles




- Slides: 19

Quantum Physics Module Summary Elliott

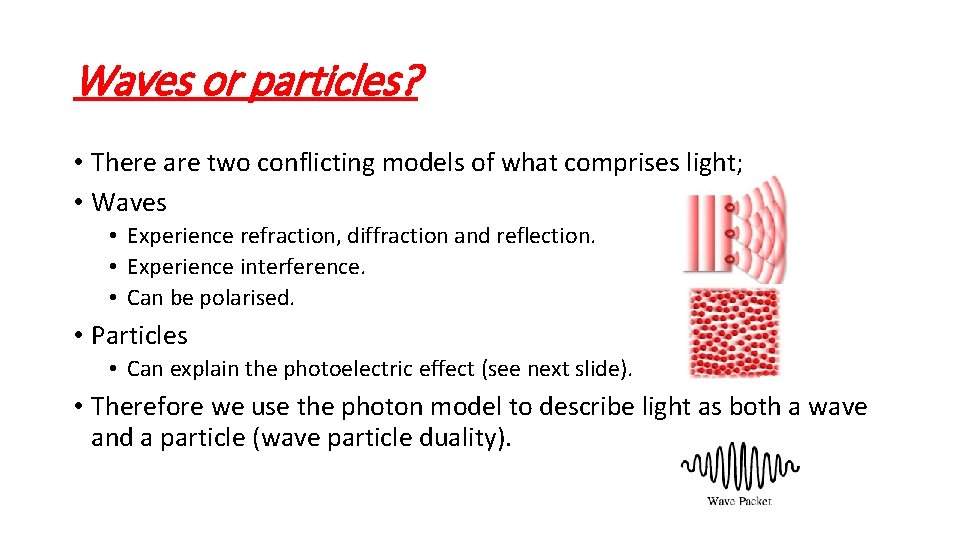
Waves or particles? • There are two conflicting models of what comprises light; • Waves • Experience refraction, diffraction and reflection. • Experience interference. • Can be polarised. • Particles • Can explain the photoelectric effect (see next slide). • Therefore we use the photon model to describe light as both a wave and a particle (wave particle duality).

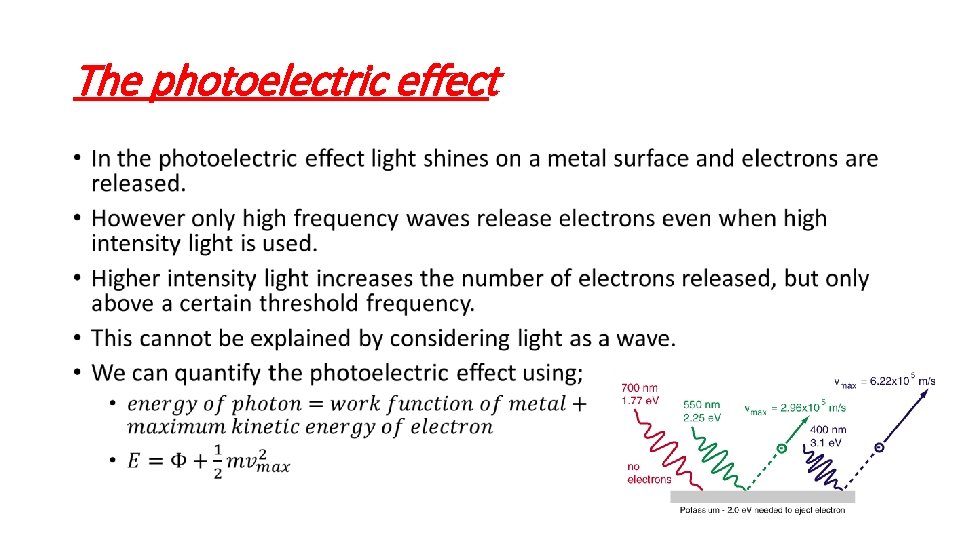
The photoelectric effect •

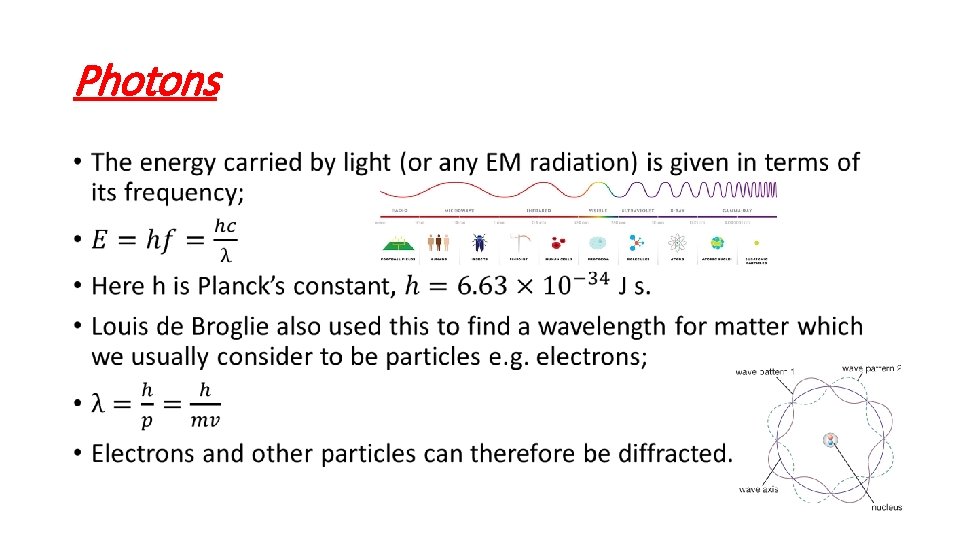
Photons •

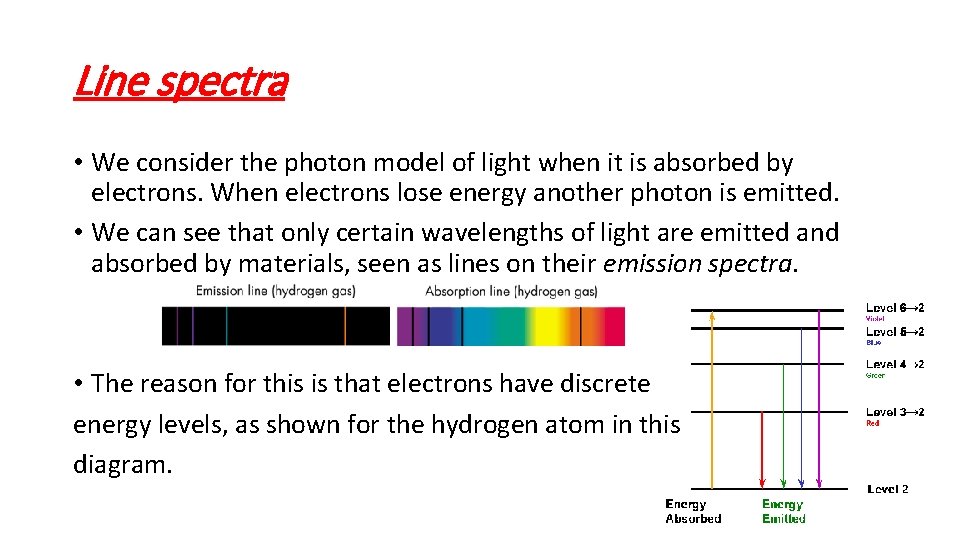
Line spectra • We consider the photon model of light when it is absorbed by electrons. When electrons lose energy another photon is emitted. • We can see that only certain wavelengths of light are emitted and absorbed by materials, seen as lines on their emission spectra. • The reason for this is that electrons have discrete energy levels, as shown for the hydrogen atom in this diagram.

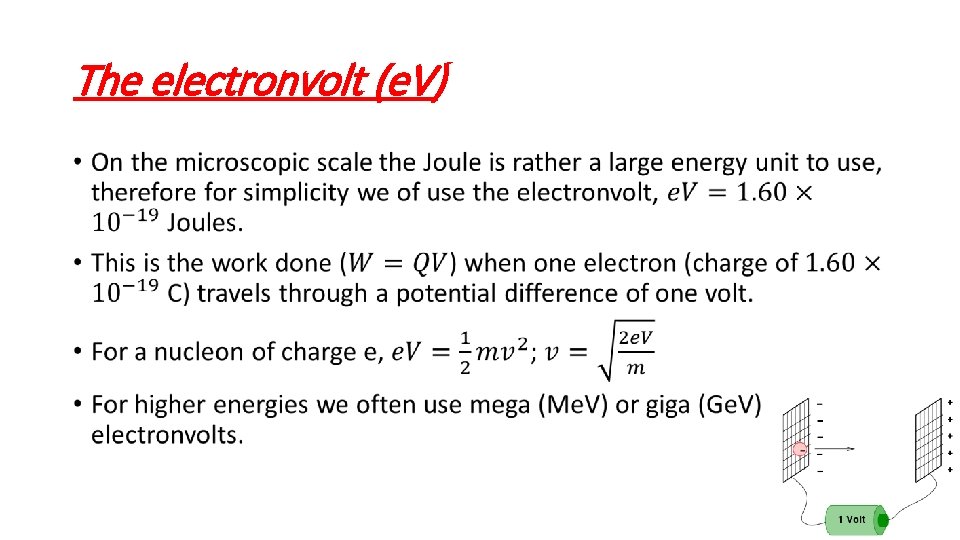
The electronvolt (e. V) •

The atom • The idea of the atom goes back to the ancient Greeks. • J. J. Thomson in 1897 discovered the electron from the study of cathode rays and even roughly guessed it’s mass. He suggested the plum-pudding model of the atom in which electrons were embedded amongst positively charged matter. • However, Ernest Rutherford noticed in 1906 that positively charged alpha particles (see later slide) mostly went straight through a thin mica sheet. He proposed that the atom is mostly empty space. • His experiments with alpha scattering led him to propose the idea of a tiny central nucleus, about 80000 times smaller than the atom.

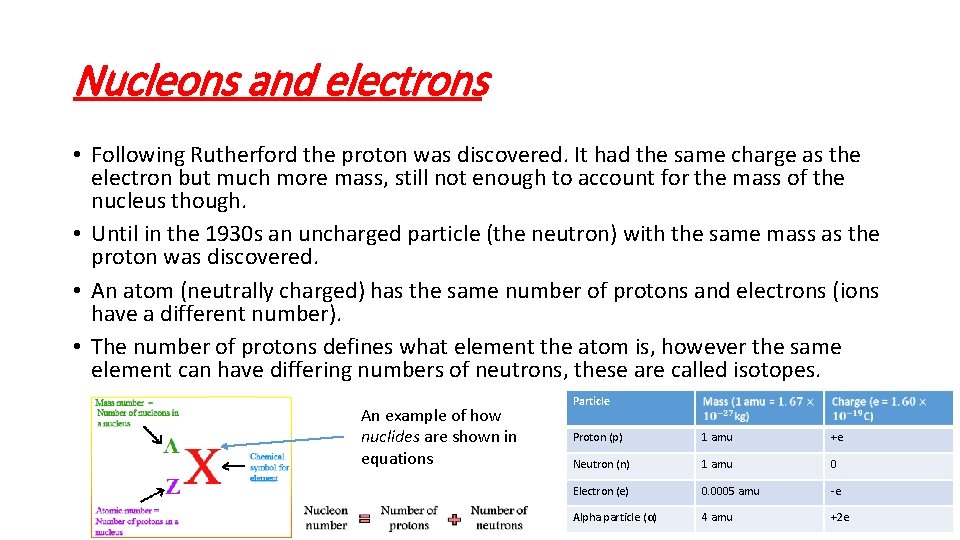
Nucleons and electrons • Following Rutherford the proton was discovered. It had the same charge as the electron but much more mass, still not enough to account for the mass of the nucleus though. • Until in the 1930 s an uncharged particle (the neutron) with the same mass as the proton was discovered. • An atom (neutrally charged) has the same number of protons and electrons (ions have a different number). • The number of protons defines what element the atom is, however the same element can have differing numbers of neutrons, these are called isotopes. An example of how nuclides are shown in equations Particle Proton (p) 1 amu +e Neutron (n) 1 amu 0 Electron (e) 0. 0005 amu -e Alpha particle (α) 4 amu +2 e

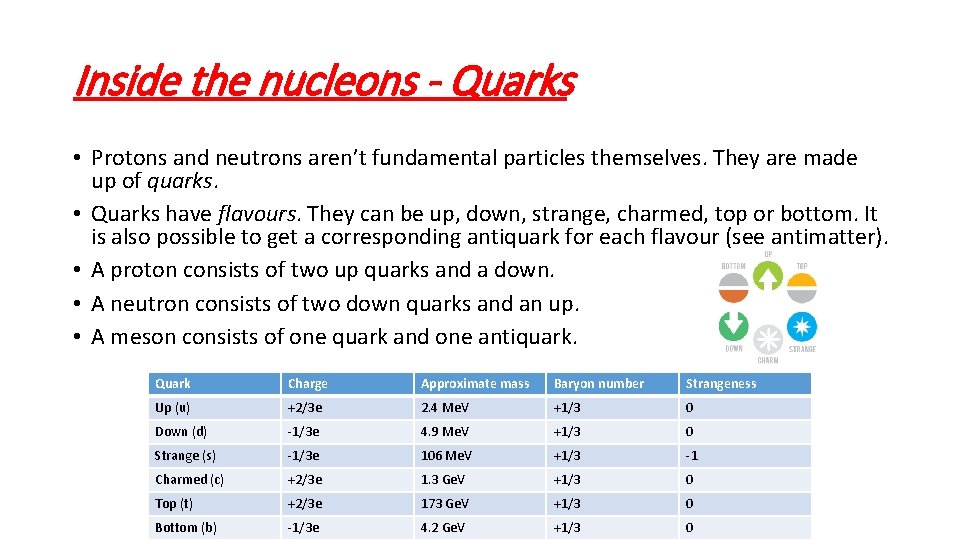
Inside the nucleons - Quarks • Protons and neutrons aren’t fundamental particles themselves. They are made up of quarks. • Quarks have flavours. They can be up, down, strange, charmed, top or bottom. It is also possible to get a corresponding antiquark for each flavour (see antimatter). • A proton consists of two up quarks and a down. • A neutron consists of two down quarks and an up. • A meson consists of one quark and one antiquark. Quark Charge Approximate mass Baryon number Strangeness Up (u) +2/3 e 2. 4 Me. V +1/3 0 Down (d) -1/3 e 4. 9 Me. V +1/3 0 Strange (s) -1/3 e 106 Me. V +1/3 -1 Charmed (c) +2/3 e 1. 3 Ge. V +1/3 0 Top (t) +2/3 e 173 Ge. V +1/3 0 Bottom (b) -1/3 e 4. 2 Ge. V +1/3 0

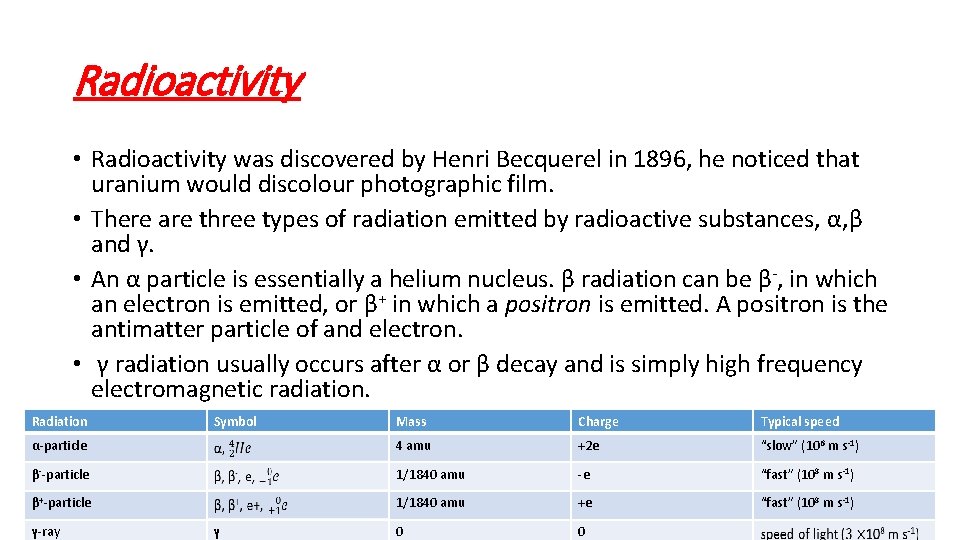
Radioactivity • Radioactivity was discovered by Henri Becquerel in 1896, he noticed that uranium would discolour photographic film. • There are three types of radiation emitted by radioactive substances, α, β and γ. • An α particle is essentially a helium nucleus. β radiation can be β-, in which an electron is emitted, or β+ in which a positron is emitted. A positron is the antimatter particle of and electron. • γ radiation usually occurs after α or β decay and is simply high frequency electromagnetic radiation. Radiation Mass Charge Typical speed α-particle 4 amu +2 e “slow” (106 m s-1) β--particle 1/1840 amu -e “fast” (108 m s-1) β+-particle 1/1840 amu +e “fast” (108 m s-1) 0 0 γ-ray Symbol γ

Antimatter • All known particles can have an antiparticle, which is a particle of the same mass but opposite properties such as charge. • If a particle collides with its antiparticle they annihilate each other to form a proton of pure energy. • In pair production the opposite is true, a high energy photon can spontaneously become a particle and its antiparticle providing their rest masses are less than the energy of the photo (see E=mc 2). The remaining energy becomes the kinetic energy of the particles.

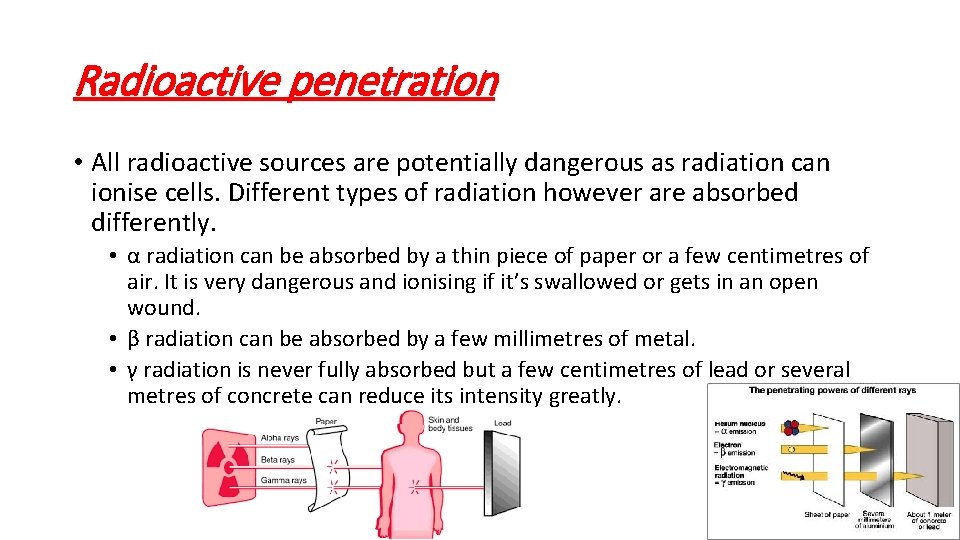
Radioactive penetration • All radioactive sources are potentially dangerous as radiation can ionise cells. Different types of radiation however are absorbed differently. • α radiation can be absorbed by a thin piece of paper or a few centimetres of air. It is very dangerous and ionising if it’s swallowed or gets in an open wound. • β radiation can be absorbed by a few millimetres of metal. • γ radiation is never fully absorbed but a few centimetres of lead or several metres of concrete can reduce its intensity greatly.

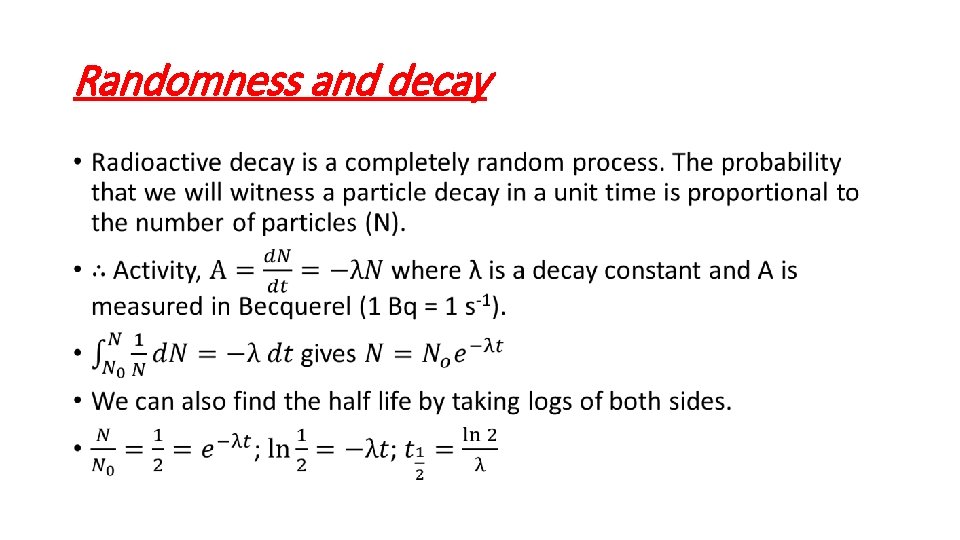
Randomness and decay •

Neutrinos • In beta decay it was noticed around 1930 that momentum as well as energy must be conserved. Wolfgang Pauli therefore predicted that another particle must be emitted. • This was called the neutrino (or electron neutrino) in beta plus decay or the electron antineutrino in beta minus. • They have negligible rest mass and are particles of no charge but pure energy. Therefore they rarely interact at all and are difficult to detect though a huge number pass through us everyday. They were first observed in 1956. A neutrino detector

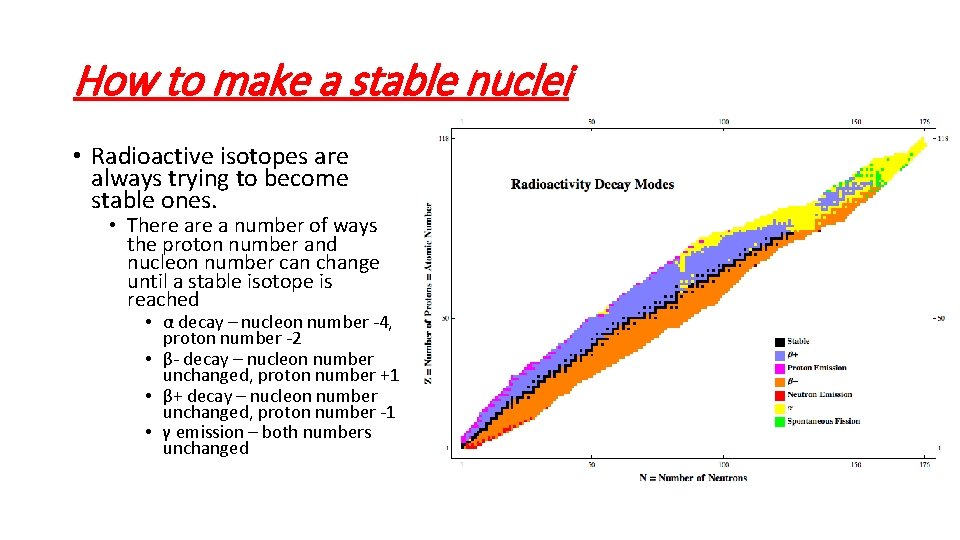
How to make a stable nuclei • Radioactive isotopes are always trying to become stable ones. • There a number of ways the proton number and nucleon number can change until a stable isotope is reached • α decay – nucleon number -4, proton number -2 • β- decay – nucleon number unchanged, proton number +1 • β+ decay – nucleon number unchanged, proton number -1 • γ emission – both numbers unchanged

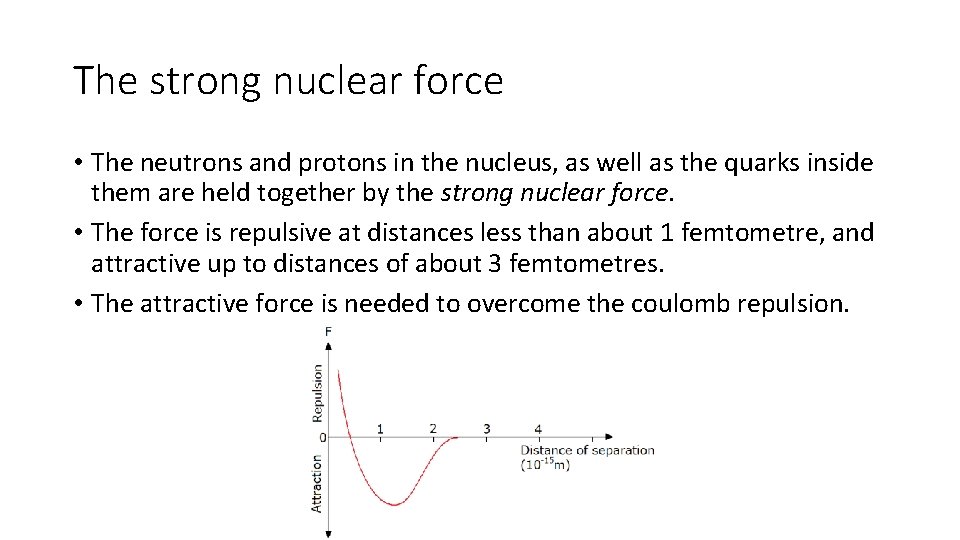
The strong nuclear force • The neutrons and protons in the nucleus, as well as the quarks inside them are held together by the strong nuclear force. • The force is repulsive at distances less than about 1 femtometre, and attractive up to distances of about 3 femtometres. • The attractive force is needed to overcome the coulomb repulsion.

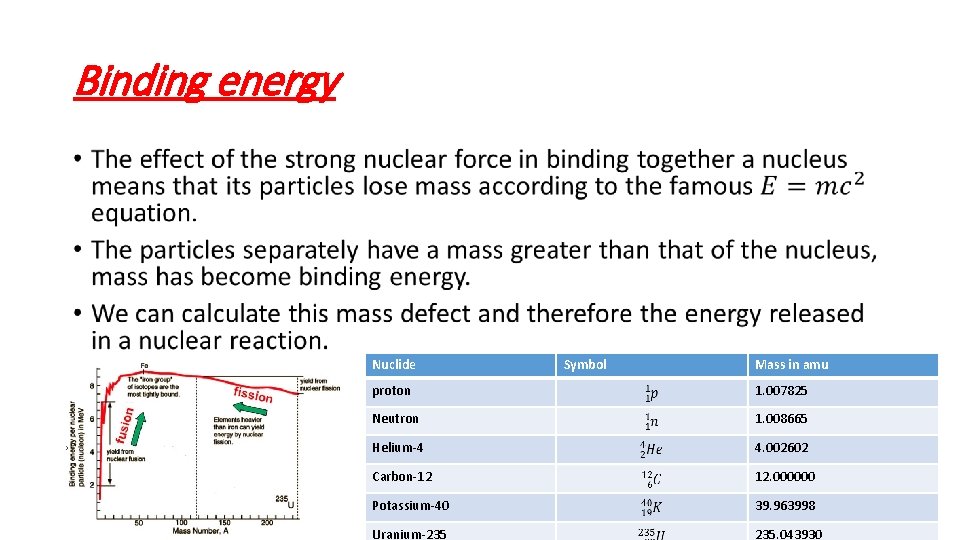
Binding energy • Nuclide Symbol Mass in amu proton 1. 007825 Neutron 1. 008665 Helium-4 4. 002602 Carbon-12 12. 000000 Potassium-40 39. 963998 Uranium-235 235. 043930

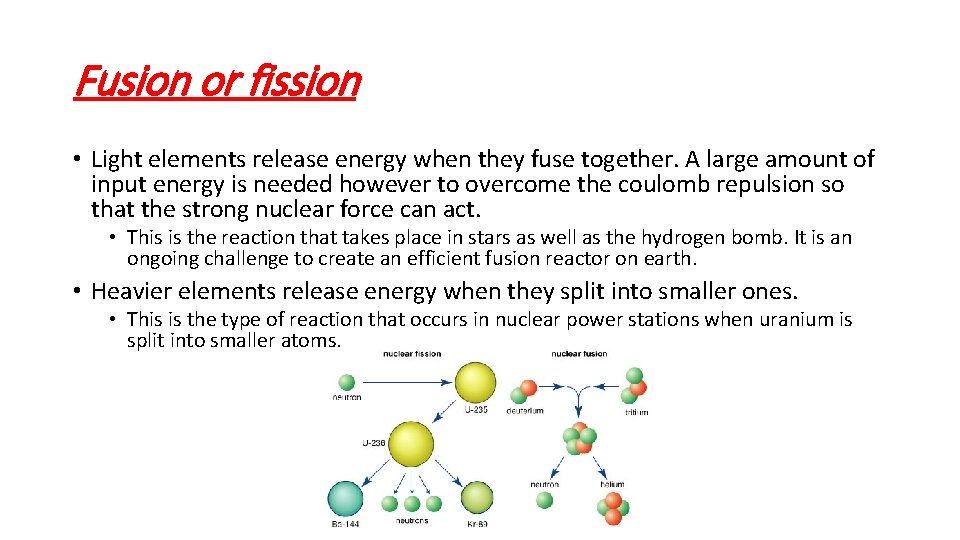
Fusion or fission • Light elements release energy when they fuse together. A large amount of input energy is needed however to overcome the coulomb repulsion so that the strong nuclear force can act. • This is the reaction that takes place in stars as well as the hydrogen bomb. It is an ongoing challenge to create an efficient fusion reactor on earth. • Heavier elements release energy when they split into smaller ones. • This is the type of reaction that occurs in nuclear power stations when uranium is split into smaller atoms.

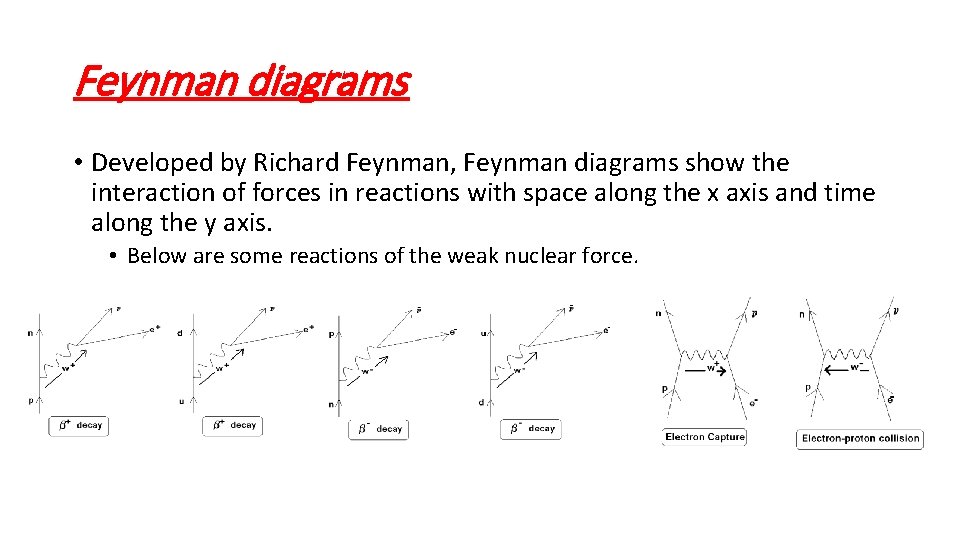
Feynman diagrams • Developed by Richard Feynman, Feynman diagrams show the interaction of forces in reactions with space along the x axis and time along the y axis. • Below are some reactions of the weak nuclear force.