Quantum Numbers What are they n n Characterization

Quantum Numbers

What are they? n n Characterization of the orbital that an electron occupies Describes the following: n n distance from the nucleus shape position with respect to the 3 dimensional axis direction of spin of the electron

What are orbitals? n Area to that an electron pair can reside in n n There are four main “shapes” for orbitals Orbitals can be found in shells which are main energy levels n aka the rings or orbits around a nucleus
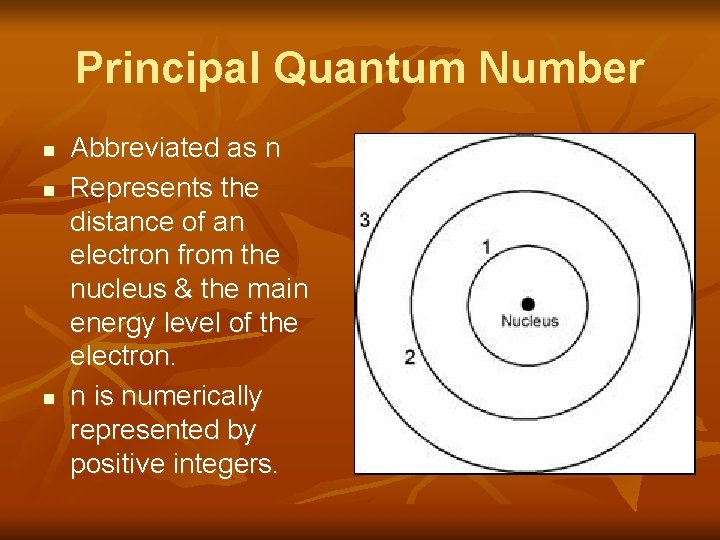
Principal Quantum Number n n n Abbreviated as n Represents the distance of an electron from the nucleus & the main energy level of the electron. n is numerically represented by positive integers.
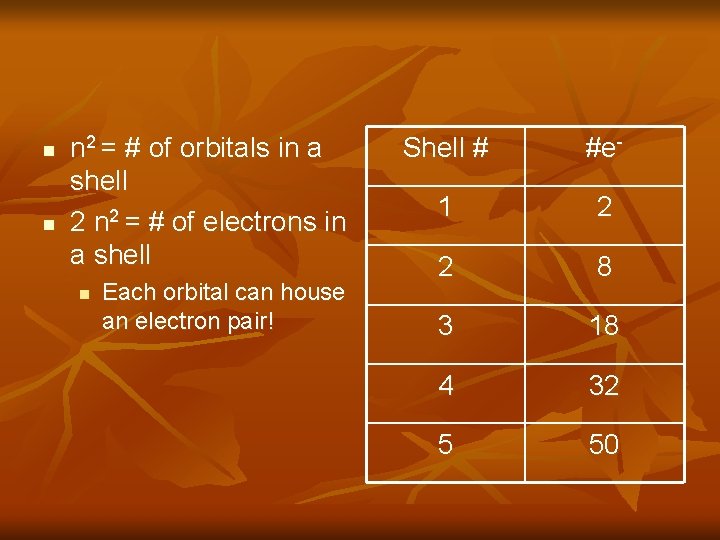
n n n 2 = # of orbitals in a shell 2 n 2 = # of electrons in a shell n Each orbital can house an electron pair! Shell # #e- 1 2 2 8 3 18 4 32 5 50
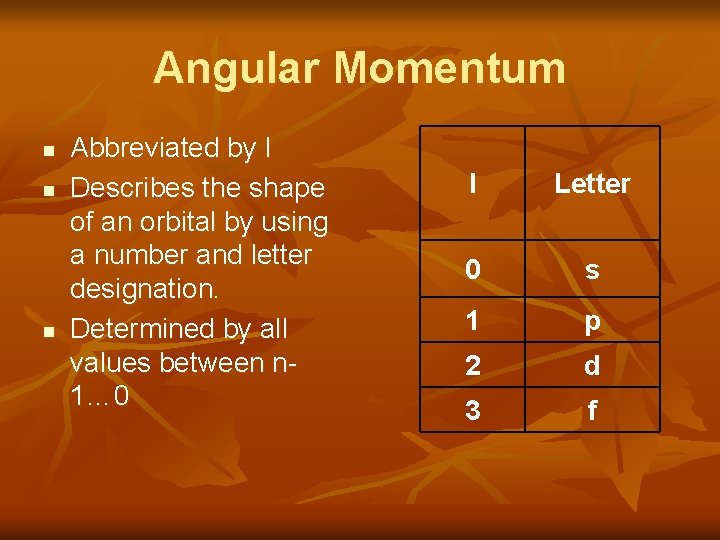
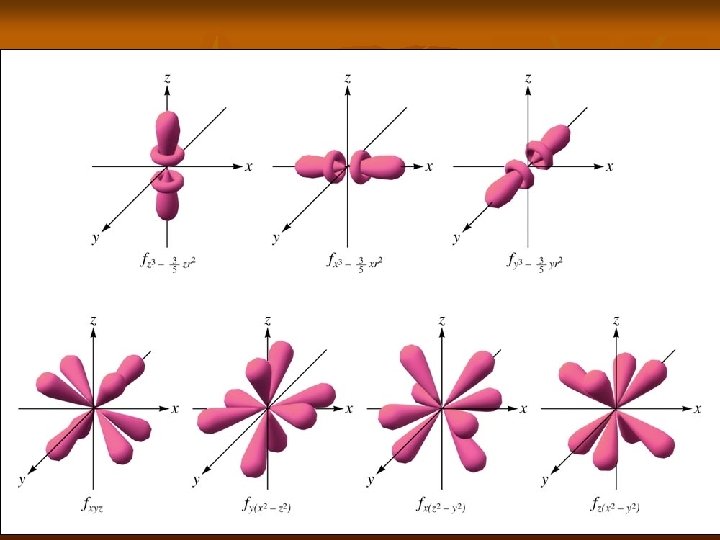
Angular Momentum n n n Abbreviated by l Describes the shape of an orbital by using a number and letter designation. Determined by all values between n 1… 0 l Letter 0 s 1 p 2 d 3 f

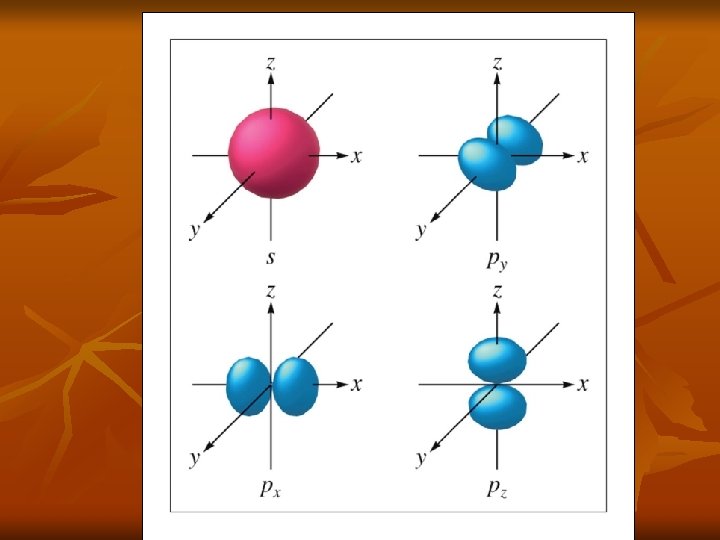
Shapes n n s orbital is spherical p orbital looks like a dumbell d orbitals look like 2 dumbells f orbitals look like flowers
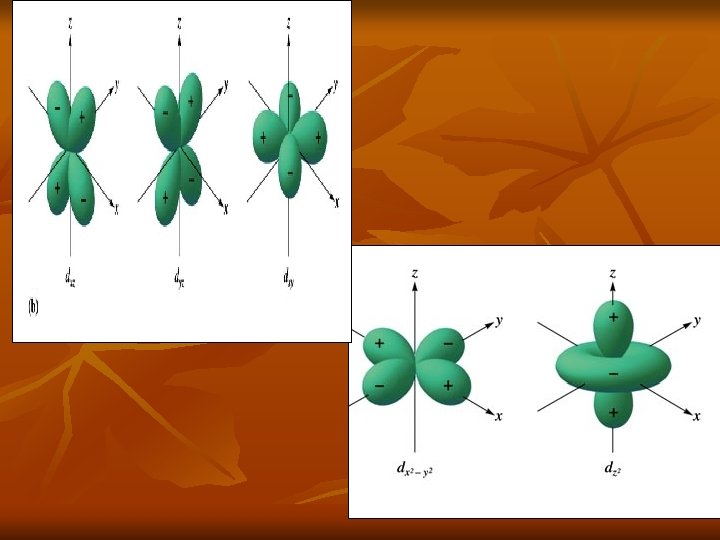

Magnetic Quantum Number n n n Abbreviated by ml Represents the 3 dimensional orientation of an orbital Assumes all values –l… 0…+l
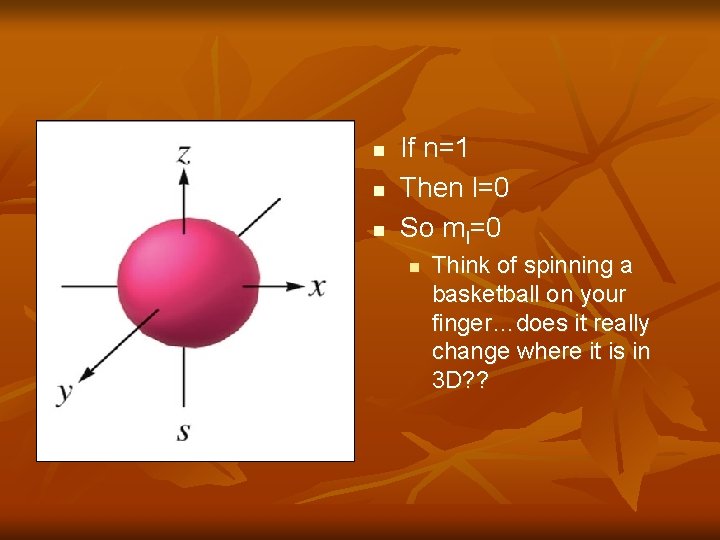
n n n If n=1 Then l=0 So ml=0 n Think of spinning a basketball on your finger…does it really change where it is in 3 D? ?
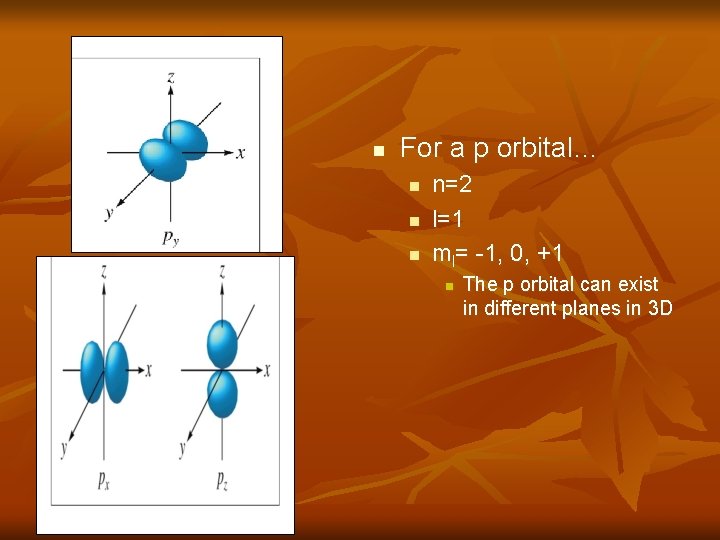
n For a p orbital… n n=2 l=1 ml= -1, 0, +1 n The p orbital can exist in different planes in 3 D

Spin Quantum Number n n Abbreviated by ms Uses +1/2 or -1/2 Indicates the direction of spin of an electron Electrons in the same orbital have opposite spins!
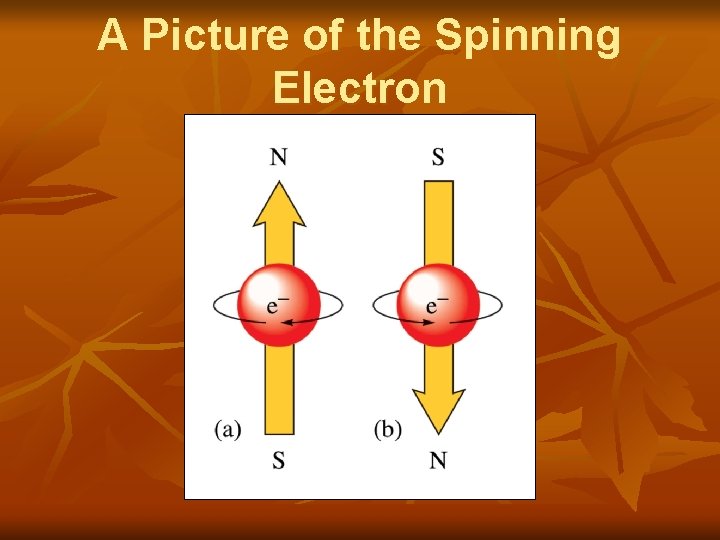
A Picture of the Spinning Electron

How do we determine QN’s? n Must use 3 main rules and determine the electron configuration!

Aufbau Principle n Electrons must enter the lowest shell and orbital first!

Hund’s Rule n orbitals of equal energy will be occupied by one electron before a second one may enter n No one can have seconds until everyone has gone through once!!

Pauli’s Exclusion Principle n No 2 electrons in the same orbital can have the same 4 Quantum Numbers!! n Don’t worry because each will have an opposite spin!!
- Slides: 19