Quantum Numbers THE FOUR QUANTUM NUMBERS Quantum Numbers

Quantum Numbers

THE FOUR QUANTUM NUMBERS Quantum Numbers numbers that describe the quantum mechanical properties of orbitals. 1) Principal Quantum Number 2) Secondary Quantum Number 3) Magnetic Quantum Number 4) Spin Quantum Number

THE PRINCIPAL QUANTUM NUMBER (n) ◦ Energy levels in atoms are sometimes called shells. Bohr called the shell number, the principal quantum number. ◦ The Principal Quantum Number ◦ The quantum number that describes the size and energy of an atomic orbital. ◦ It has whole number values (1, 2, 3 and so on) ◦ As n increases the energy required for an electron to occupy that orbital increases. Each successive orbital is larger
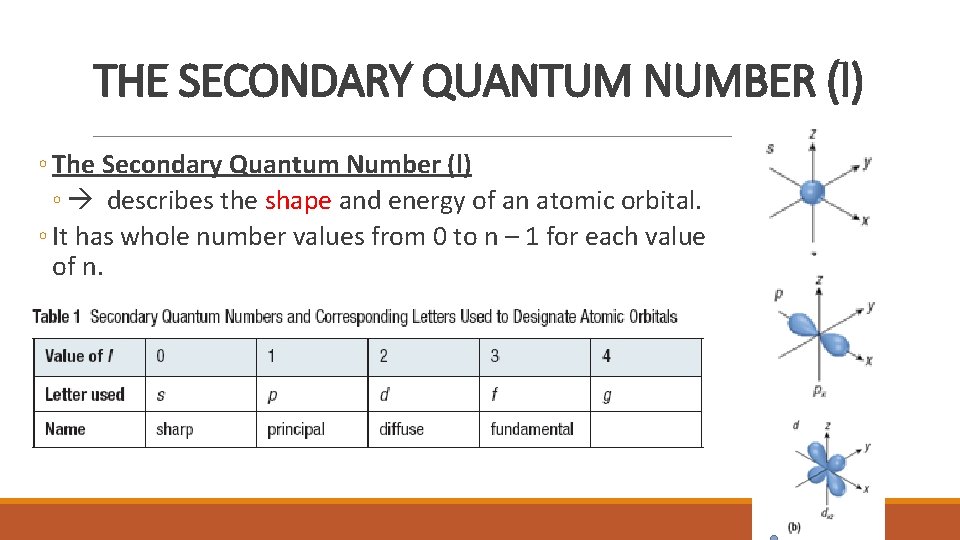
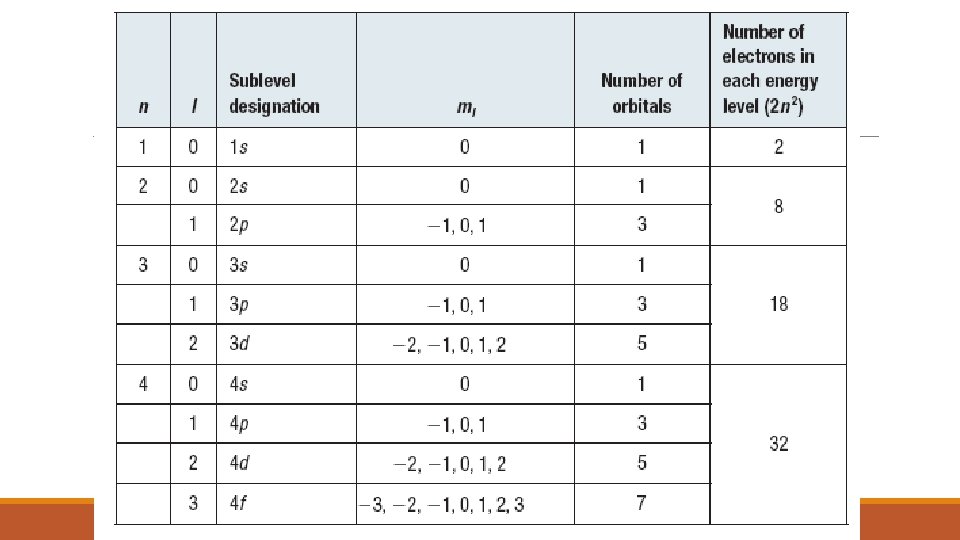
THE SECONDARY QUANTUM NUMBER (l) ◦ The Secondary Quantum Number (l) ◦ describes the shape and energy of an atomic orbital. ◦ It has whole number values from 0 to n – 1 for each value of n.

THE MAGNETIC QUANTUM NUMBER (ml) ◦ The Magnetic Quantum Number (ml) ◦ the quantum number that describes the orientation of an atomic orbital in space relative to the other orbitals in the atom. ◦ It has whole number value between +l and –l including 0.
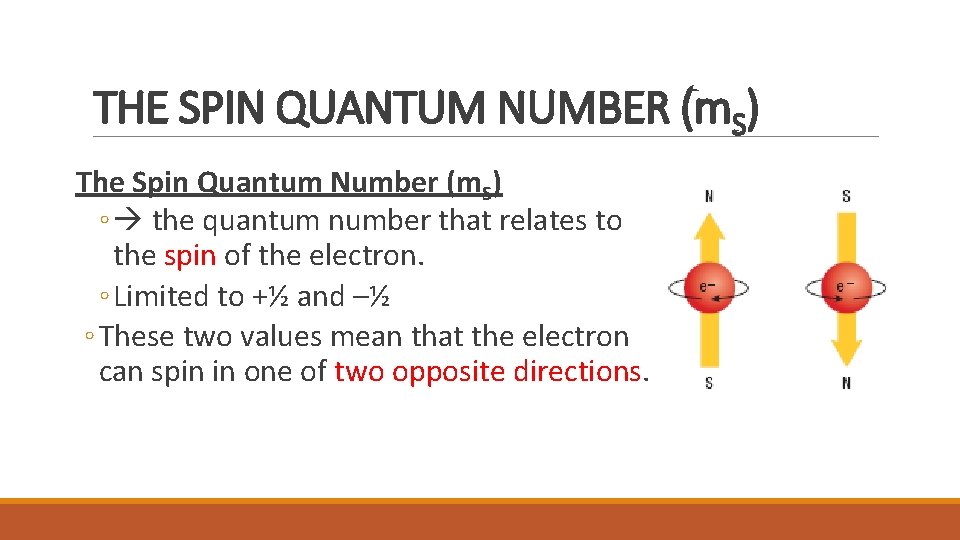
THE SPIN QUANTUM NUMBER (m. S) The Spin Quantum Number (m. S) ◦ the quantum number that relates to the spin of the electron. ◦ Limited to +½ and –½ ◦ These two values mean that the electron can spin in one of two opposite directions.

THE PAULI EXCLUSION PRINCIPLE ◦ The Pauli Exclusion Principle ◦ In a given atom, no two electrons can have the same set of four quantum numbers ( n, l, ml, and ms )

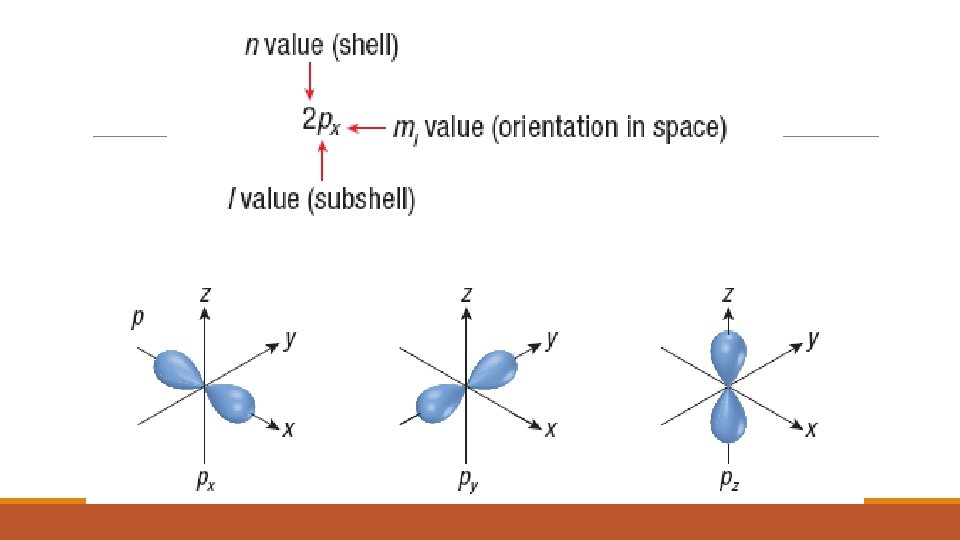
SHAPES AND ORIENTATION OF ORBTIALS Each orbital has a unique probability distribution, shape and orientation. ◦ S orbitals ◦ are spherical in shape and they become larger as the value of n increases. ◦ P orbitals – ◦ are not spherical like the s orbitals but have two lobes separated by a node at the nucleus. ◦ the p orbitals can exist in any of the three dimensions and are labeled using the xyz coordinate system along which the lobes lie. ◦ Ex. 2 p orbital with its lobes centered along the x axis is the 2 px orbital.
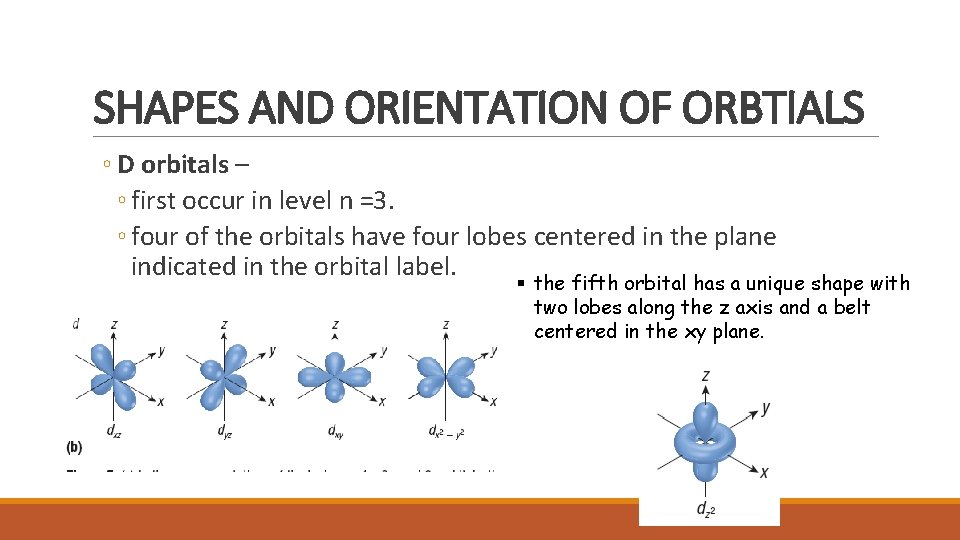
SHAPES AND ORIENTATION OF ORBTIALS ◦ D orbitals – ◦ first occur in level n =3. ◦ four of the orbitals have four lobes centered in the plane indicated in the orbital label. the fifth orbital has a unique shape with two lobes along the z axis and a belt centered in the xy plane.
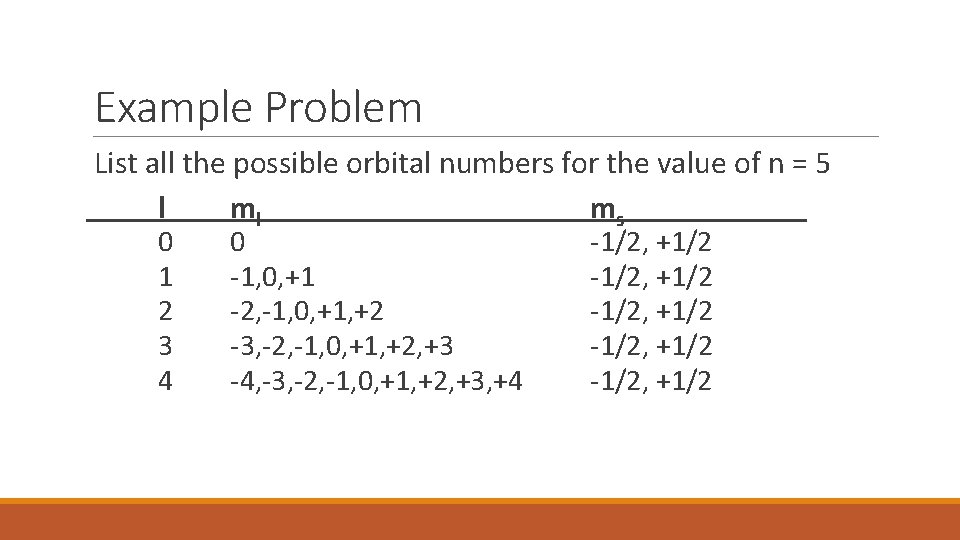
Example Problem List all the possible orbital numbers for the value of n = 5 l ml ms 0 1 2 3 4 0 -1, 0, +1 -2, -1, 0, +1, +2 -3, -2, -1, 0, +1, +2, +3 -4, -3, -2, -1, 0, +1, +2, +3, +4 -1/2, +1/2 -1/2, +1/2

Homework



- Slides: 16