Quantum Numbers Quantum Numbers specify the properties of

Quantum Numbers • Quantum Numbers specify the properties of atomic orbitals and their electrons • There are four quantum numbers: • principal quantum number • orbital quantum number • magnetic quantum number • spin quantum number

Principal Quantum Number • The principal quantum number (n) specifies the main energy levels around the nucleus • As n increases, the distance from the nucleus increases (So as the numbers become larger the levels become further from the nucleus) • Currently the values for n are 1 to 7 • These orbitals are called subshells or sublevels • The four most common Principal quantum numbers are given letter abbreviations s, p, d, f

Orbital Quantum Number • Orbital Quantum Number ( l ) indicates the shape of the orbital where the electron can be found

“s” Orbital
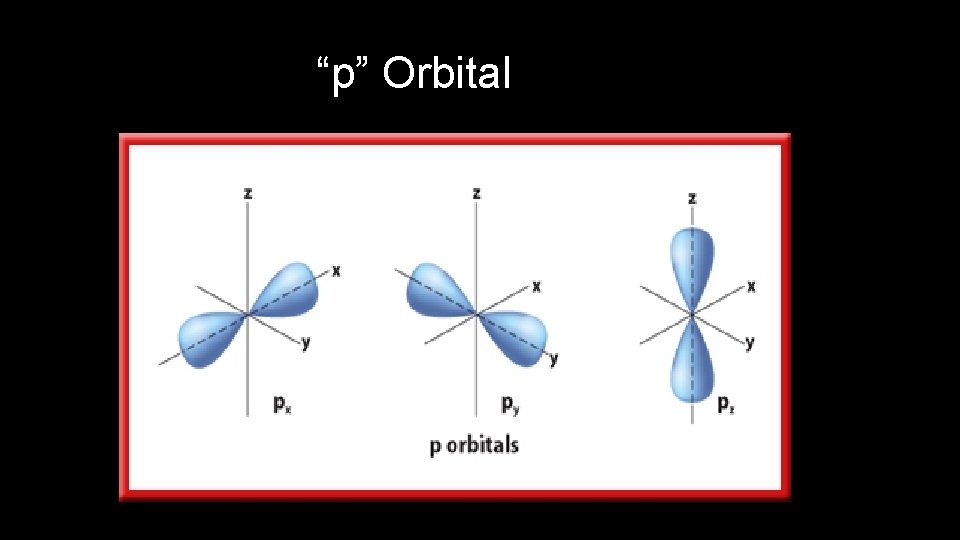
“p” Orbital
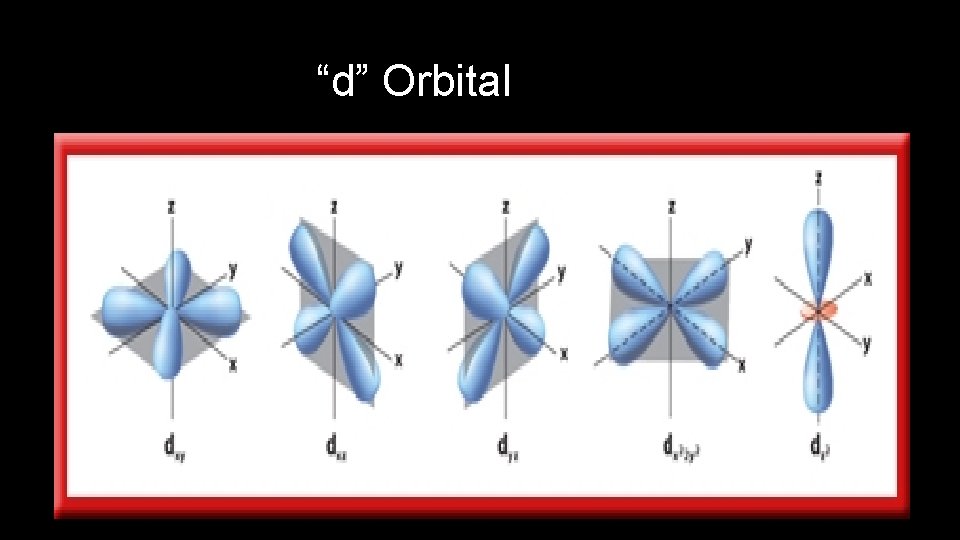
“d” Orbital
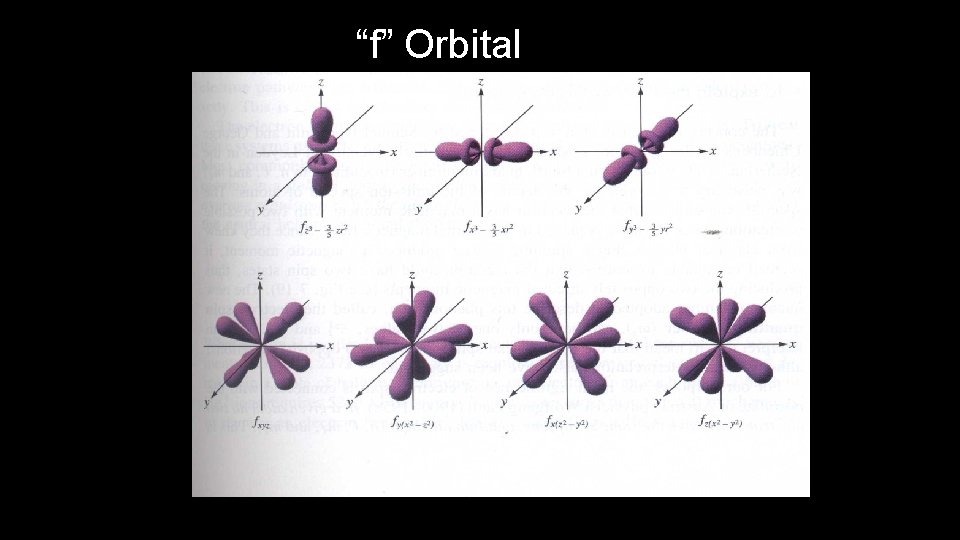
“f” Orbital

Magnetic Quantum Number • 3. Magnetic Quantum Number (ml ): indicates the orientation of an orbital about the nucleus • it tells which axis that sublevel is located on (x, y, or z axis)

Spin Quantum Number • The Spin Quantum Number (s) is a value (of 1/2) that describes the angular momentum of an electron. An electron spins around an axis and has both angular momentum and orbital angular momentum. Because angular momentum is a vector, the Spin Quantum Number (s) has both a magnitude (1/2) and direction (+ or -)

Using Quantum Numbers • Quantum numbers can be used to write electron configurations • Electron configurations show electrons are most likely distributed around the nucleus

Rules for Electron Configurations • 1. Aufbau Principle: Electrons enter orbitals of lowest energy first • 2. Hund’s Rule: Orbitals of equal energy are each occupied by one electron before any one orbital is occupied by a second electron
Rules for Electron Configurations • 3. Pauli Exclusion Principle: an atomic orbital may describe at most two electrons • The maximum number of electrons in any orbital is “ 2” • No two electrons in the same atom can have the same set of four quantum numbers
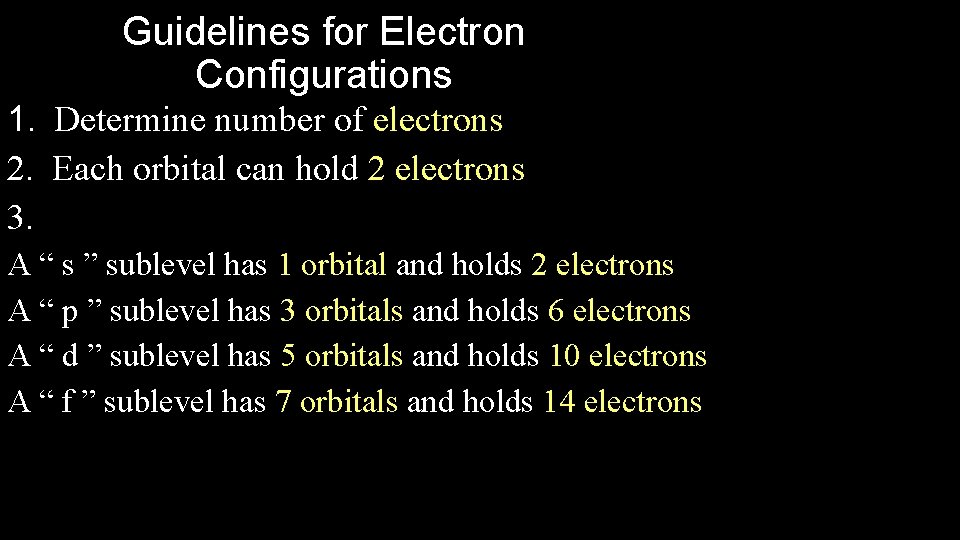
Guidelines for Electron Configurations 1. Determine number of electrons 2. Each orbital can hold 2 electrons 3. A “ s ” sublevel has 1 orbital and holds 2 electrons A “ p ” sublevel has 3 orbitals and holds 6 electrons A “ d ” sublevel has 5 orbitals and holds 10 electrons A “ f ” sublevel has 7 orbitals and holds 14 electrons
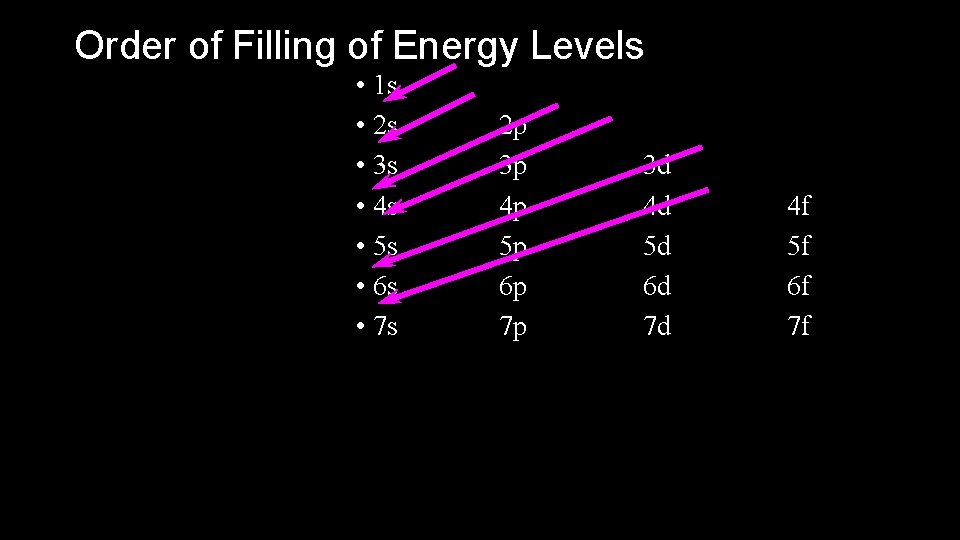
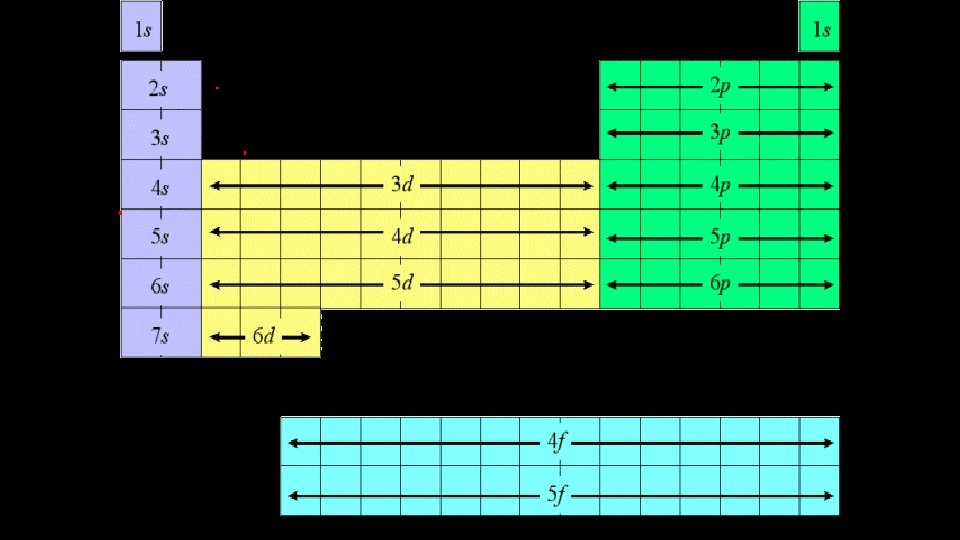
Order of Filling of Energy Levels • 1 s • 2 s • 3 s • 4 s • 5 s • 6 s • 7 s 2 p 3 p 4 p 5 p 6 p 7 p 3 d 4 d 5 d 6 d 7 d 4 f 5 f 6 f 7 f

Warm-Up • The density of a substance is 5. 25 g/m. L. What is the mass(in kilograms) of 0. 75 L of the substance?

Warm-Up • What elements are the following: • 1 s 22 p 4 1 s 22 p 63 s 23 p 64 s 23 d 7 1 s 22 p 63 s 23 p 64 s 23 d 104 p 65 s 24 d 105 p 11

Practice Long Hand Electron Configurations • Cd • B • P • Ba • As • I • Na

Review • Write the Long Hand/Expanded electron configuration for the following elements: • Sn • Ga • Y

Noble Gas Electron Configuration • Place the following Element using the Noble Gas/short hand electron configuration: • Sc • I • Ru • Fr • Fe • F

Practice Problems • Write the Noble Gas Electron Configuration for the following: • Tc • Zn • K • I • P
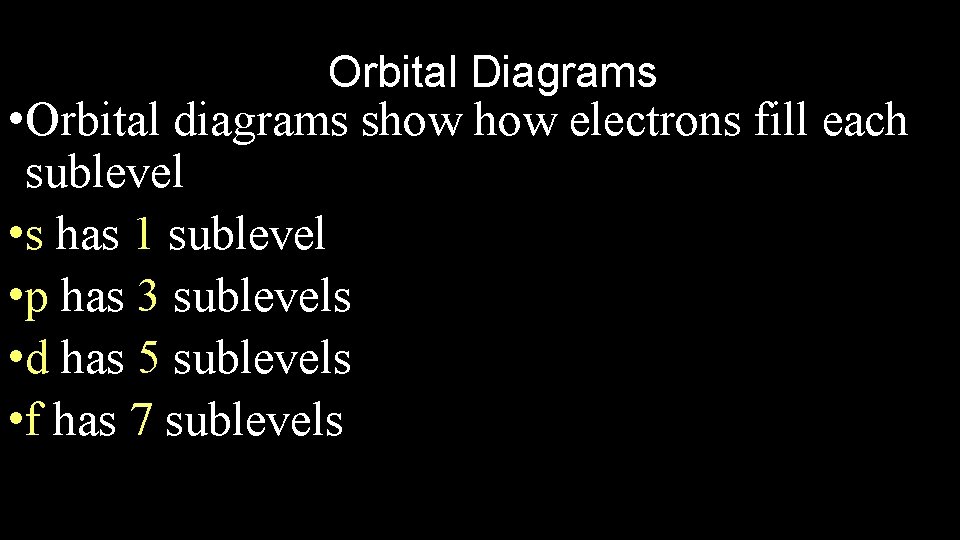
Orbital Diagrams • Orbital diagrams show electrons fill each sublevel • s has 1 sublevel • p has 3 sublevels • d has 5 sublevels • f has 7 sublevels
- Slides: 22