Quantum Numbers Quantum Mechanical Model Electron Cloud The
Quantum Numbers
Quantum – Mechanical Model (Electron Cloud) • The electron cloud is a visual representation of the most probable locations for an electron within an atom. • “Clarity through fuzziness”

Energy Levels – Sublevels - Orbitals • Electrons in an atom are within atomic orbitals which are within sublevels which are within energy levels. • Chemistry uses quantum numbers to describe these electrons.
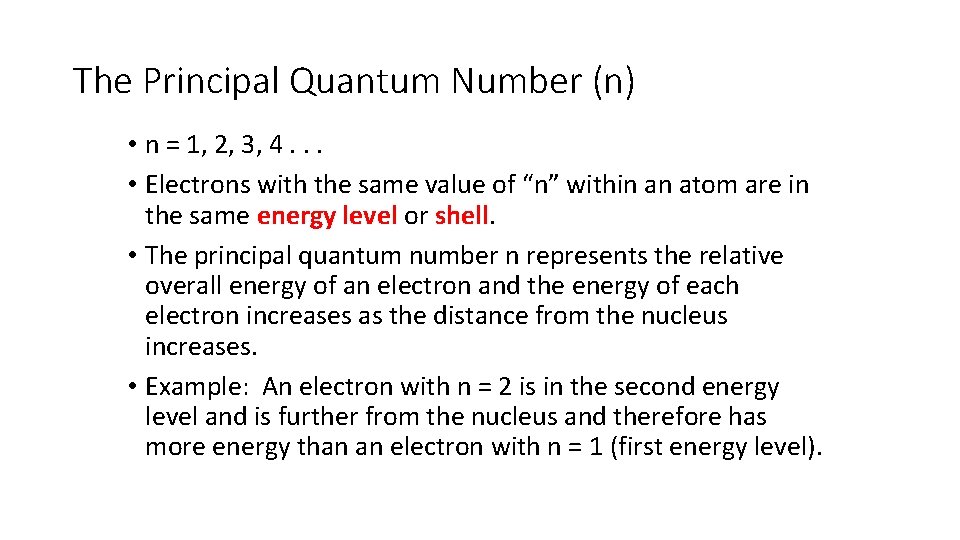
The Principal Quantum Number (n) • n = 1, 2, 3, 4. . . • Electrons with the same value of “n” within an atom are in the same energy level or shell. • The principal quantum number n represents the relative overall energy of an electron and the energy of each electron increases as the distance from the nucleus increases. • Example: An electron with n = 2 is in the second energy level and is further from the nucleus and therefore has more energy than an electron with n = 1 (first energy level).

The Maximum Number of sublevels in an Energy Level n For the 3 rd energy level n = 3 and the third energy level has 3 sublevels.
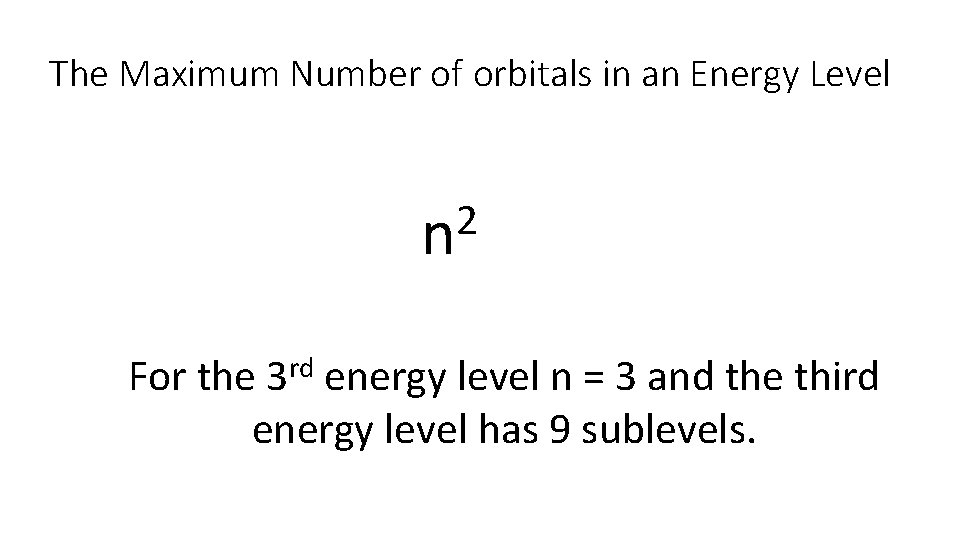
The Maximum Number of orbitals in an Energy Level 2 n For the 3 rd energy level n = 3 and the third energy level has 9 sublevels.

The Maximum Number of electrons in an Energy Level 2 2 n For the 3 rd energy level n = 3 and the third energy level can hold up to 18 electrons.

The Azimuthal Quantum Number (l) • l = 0…(n – 1). • Orbitals with the same value of “n” may have different shapes. The “l” value indicates the shape of the orbitals. • Electrons with the same value of “l” within an atom are in the same sublevel or subshell. • Example: In the fourth energy level (n = 4) there are four different orbital shapes possible designated l = 0, 1, 2 or 3.
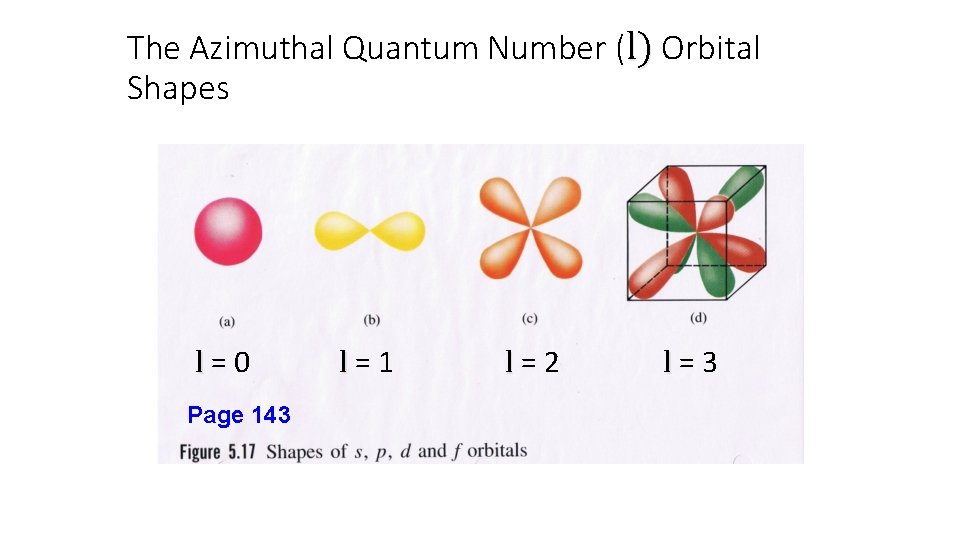
The Azimuthal Quantum Number (l) Orbital Shapes l=0 Page 143 l=1 l=2 l=3

Magnetic Quantum Number (m) • m = -l… 0…+ l. • Orbitals within an energy level with the same value of l have the same shape and energy (degenerate) but differ in their orientation. • Each possible orientation of the orbital has a specific value of “m”. • Electrons with the same value of “m” are in the same atomic orbital (the region of space that an electron is most likely to be found within an atom).

Magnetic Quantum Number (m) • Example: If l = 1 then m has three possible orientations designated: m = -1, 0 or +1. • These 3 different “m” values indicate the 3 different orbitals within a “p sublevel”.
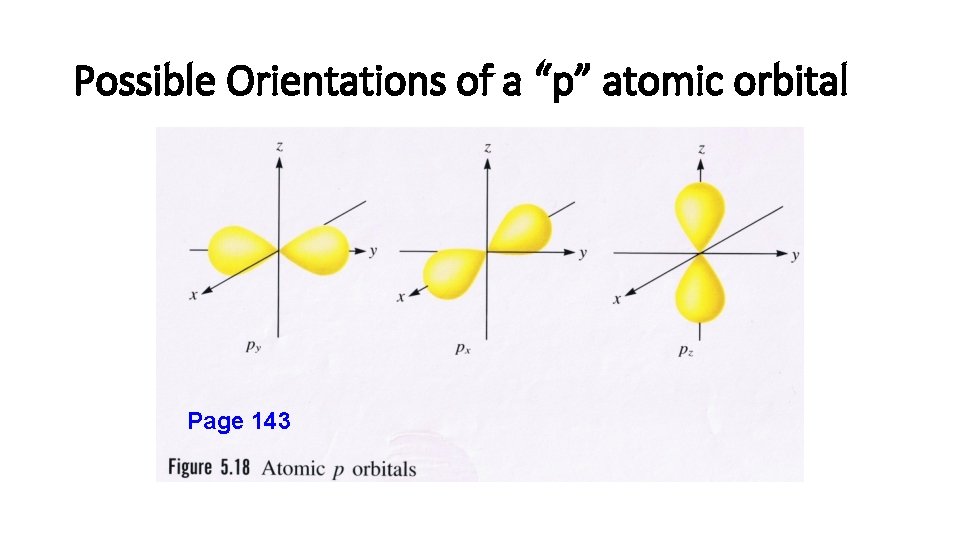
Possible Orientations of a “p” atomic orbital Page 143
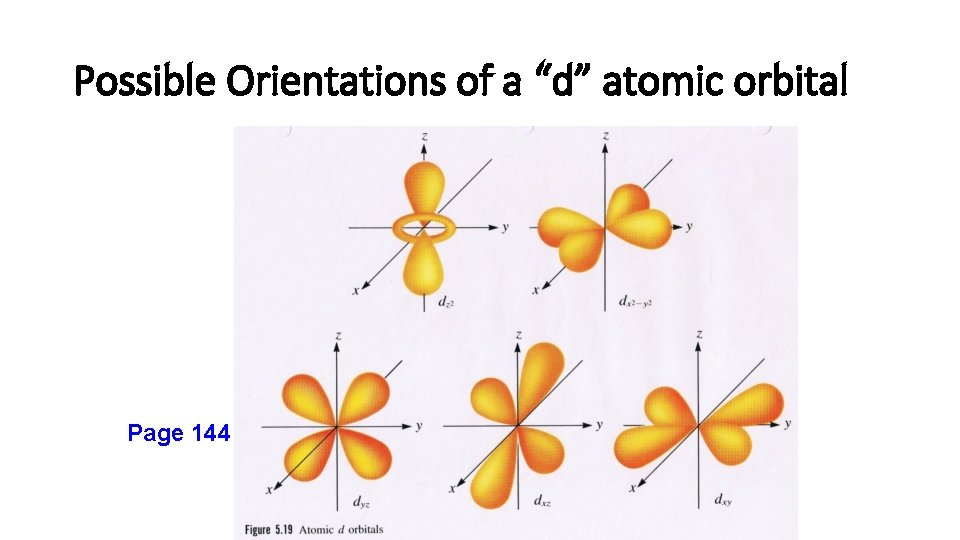
Possible Orientations of a “d” atomic orbital Page 144
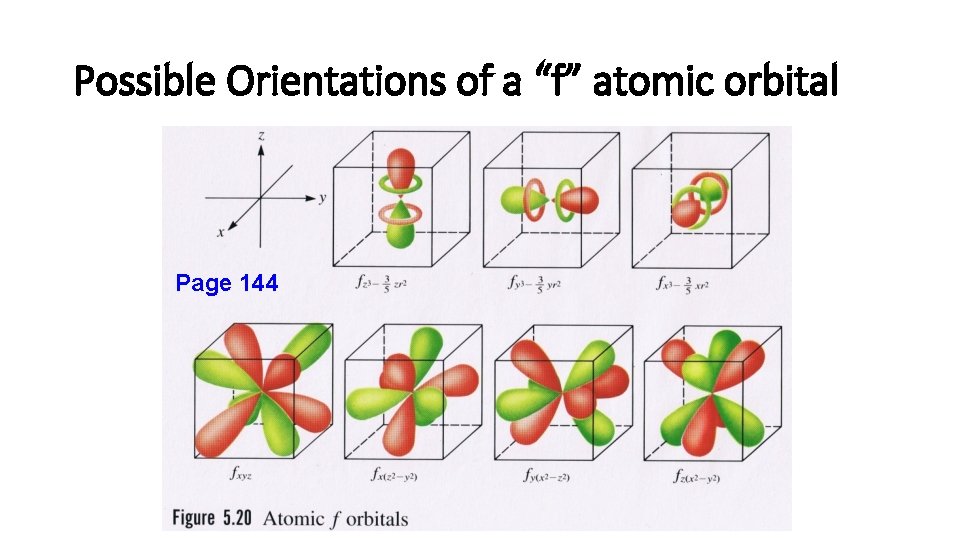
Possible Orientations of a “f” atomic orbital Page 144

Pauli Exclusion Principle • No two electrons in the same atom can have the same set of four quantum numbers.

Spin Quantum Number (s) • S = +1/2 or -1/2 • Specifies the direction of spin of the electron on its axis. • Spins are designated up or down.
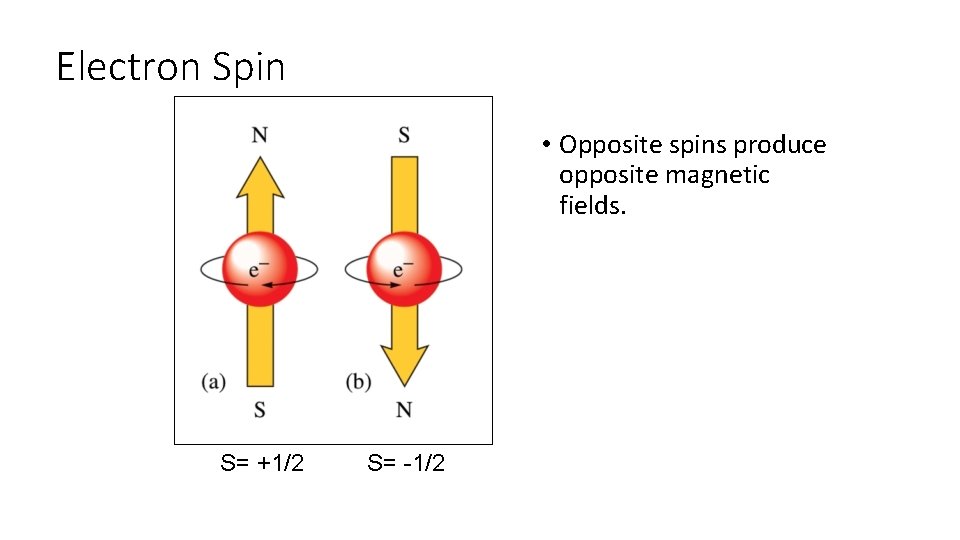
Electron Spin • Opposite spins produce opposite magnetic fields. S= +1/2 S= -1/2
- Slides: 17