QUANTUM NUMBERS Part One Terms to Know Energy

QUANTUM NUMBERS Part One

Terms to Know � Energy level/shell: describes an area that is a specific distance from the nucleus � Subshell: uniquely shaped regions at each energy level where a specific number of electrons may be found � Orbit: an area within a subshell which can hold only 2 electrons
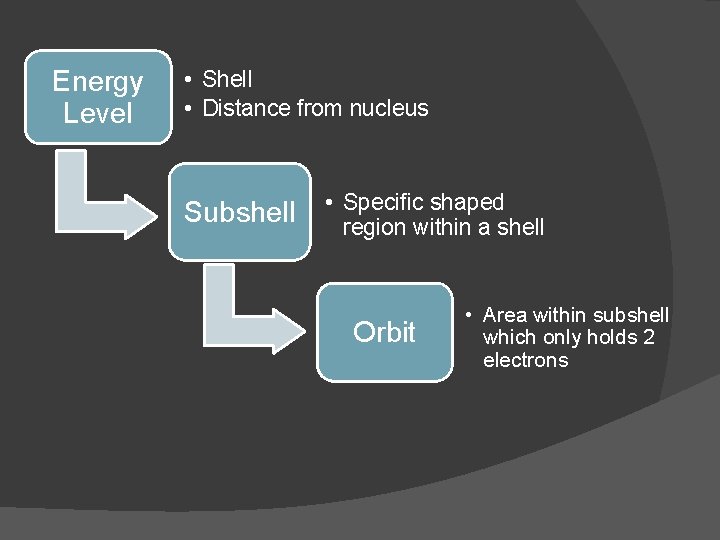
Energy Level • Shell • Distance from nucleus Subshell • Specific shaped region within a shell Orbit • Area within subshell which only holds 2 electrons

Defined � Quantum � You numbers describe electrons. can think of an electron’s quantum numbers as being that electron’s address.

Our numbers � To describe the exact location of an electron we need 4 numbers: �Principle �Angular momentum �Magnetic �Spin
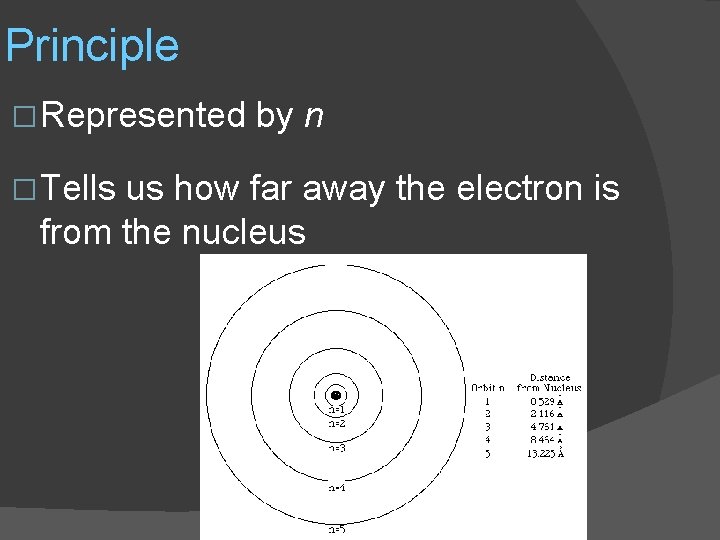
Principle � Represented � Tells by n us how far away the electron is from the nucleus
How to find principle numbers �Most elements have multiple energy levels, so they have multiple principle numbers. �Period = number of energy levels �Transition metals & inner transition metals are exceptions.
Atom = Upside down pyramid
Angular Momentum � Represented by l � Describes the shape of the subshell where the electron can be found � Energy levels can have multiple subshells
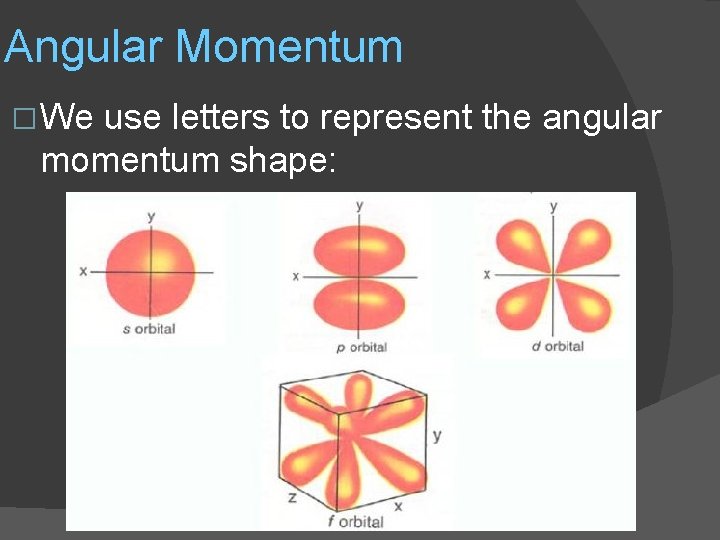
Angular Momentum � We use letters to represent the angular momentum shape:

Electrons per subshell �S subshells hold up to 2 electrons �P subshells hold up to 6 electrons �D subshells hold up to 10 electrons �F subshells hold up to 14 electrons �If each subshell contains orbits, and each orbit can hold 2 electrons, how many orbits are within each subshell?

Remember! � Hydrogen and Helium only have one energy level, but all other elements have multiple levels. � Energy levels are cumulative. � Subshell shapes are also cumulative.
- Slides: 12