QUANTUM NUMBERS ORBITALS Quantum mechanical model Mathematical equations

QUANTUM NUMBERS & ORBITALS
Quantum mechanical model • Mathematical equations to determine probability of locating an electron in an atom • Electron cloud – region of space around a nucleus with high probability of locating an electron • Windmill/ fan analogy

Orbitals • Mathematical expression • Area of atom where electrons are likely to be found • Only 2 electrons per orbital
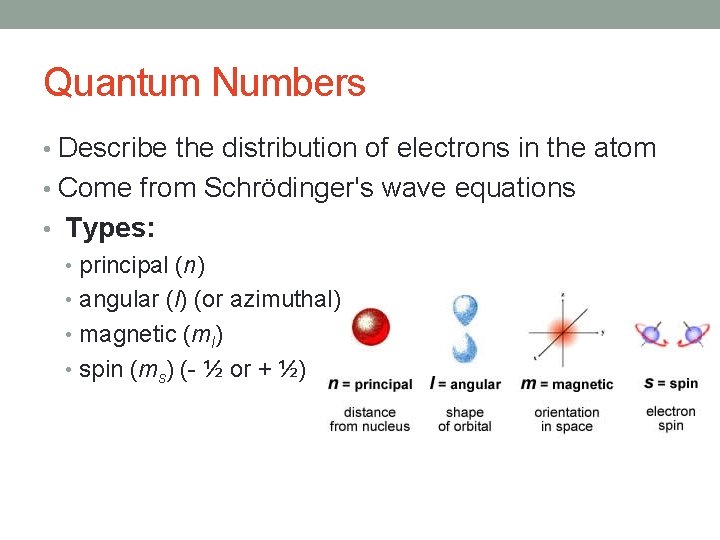
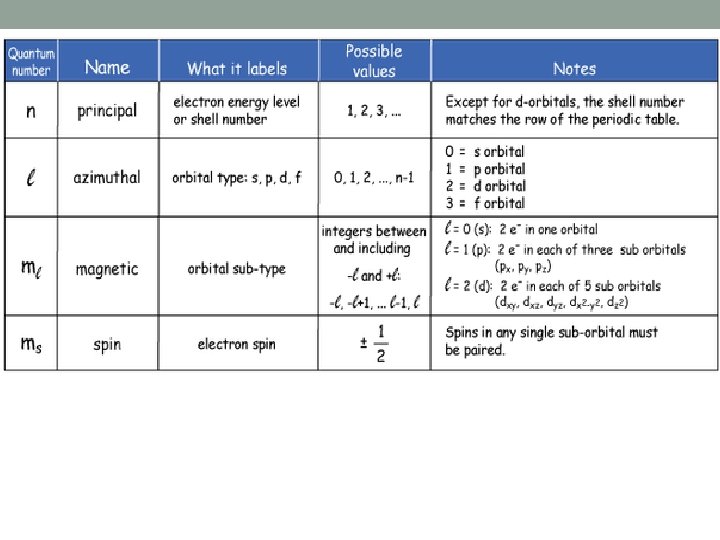
Quantum Numbers • Describe the distribution of electrons in the atom • Come from Schrödinger's wave equations • Types: • principal (n) • angular (l) (or azimuthal) • magnetic (ml) • spin (ms) (- ½ or + ½)

Principal Quantum Number (n) • Describes the size of the orbital • Distance from the nucleus • Orbitals get larger as move out from nucleus • Requires energy input to move an electron from an orbital to another orbital further out • Indirectly describes the energy of an orbital

Angular Quantum Number (l) • Describes the shape of the orbital • spherical (l = 0) • polar (l = 1) (or dumbbell) • cloverleaf (l = 2) • take on more complex shapes as the value of the angular quantum number becomes larger
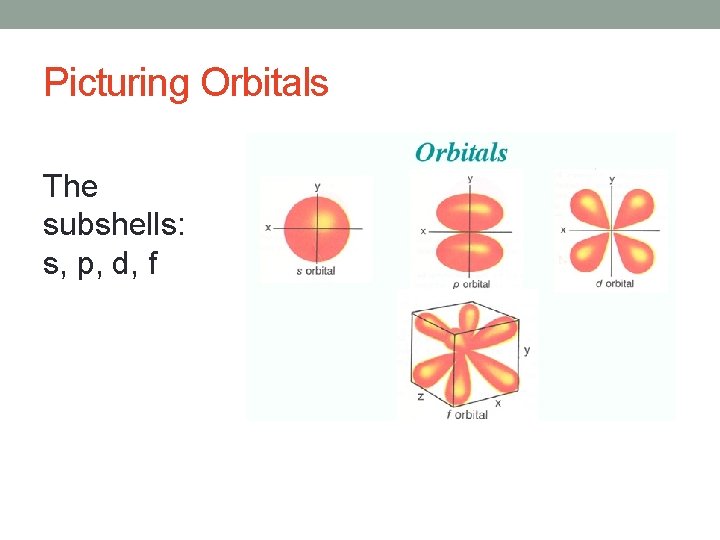
Picturing Orbitals The subshells: s, p, d, f
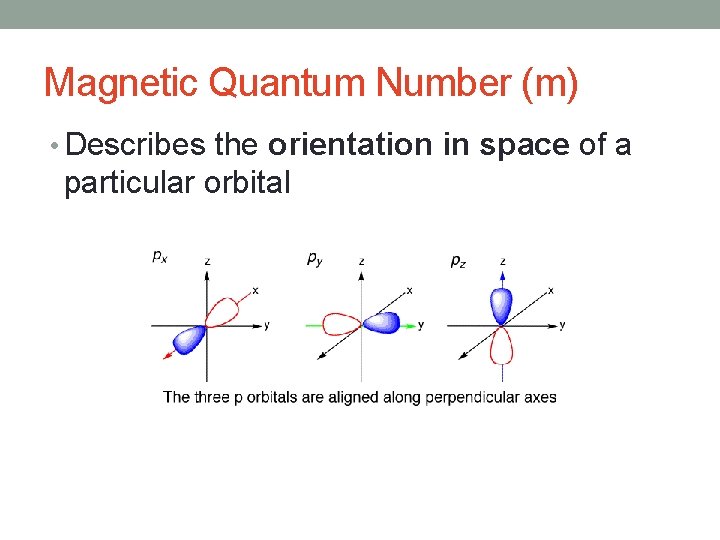
Magnetic Quantum Number (m) • Describes the orientation in space of a particular orbital
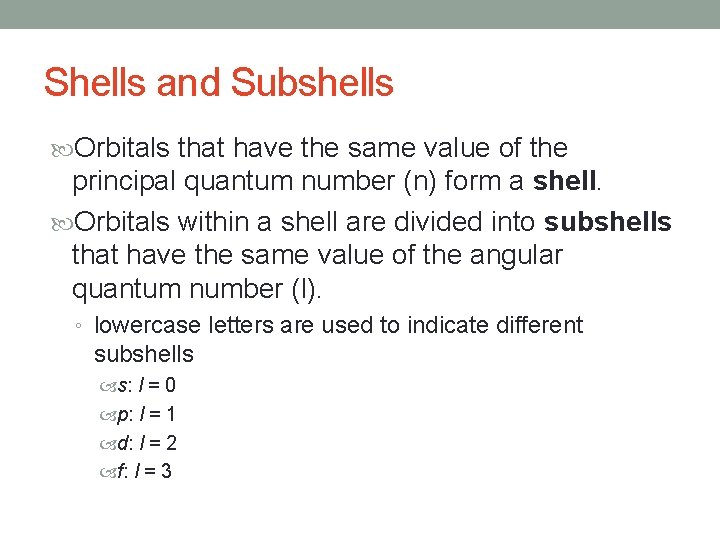
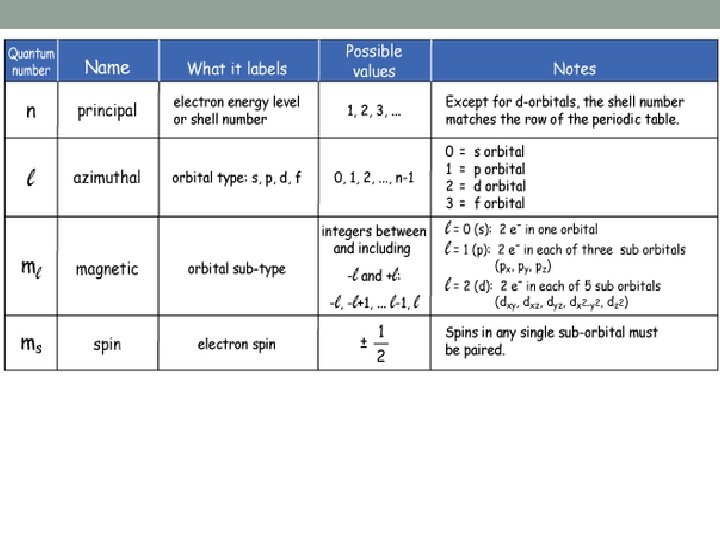
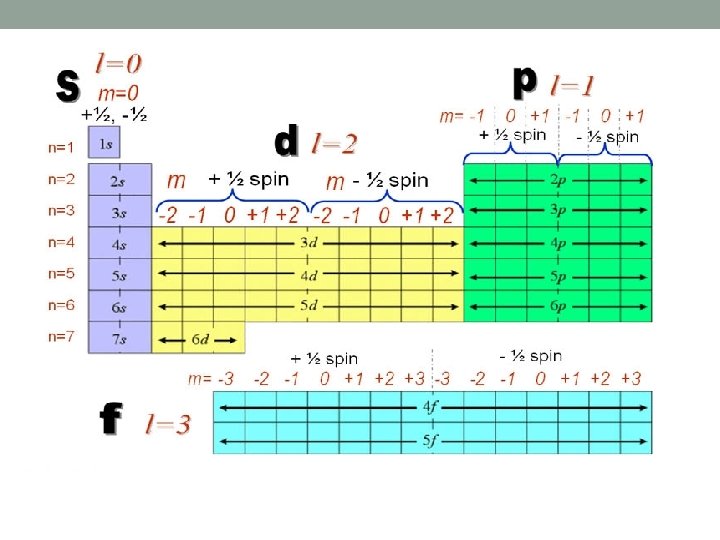
Shells and Subshells Orbitals that have the same value of the principal quantum number (n) form a shell. Orbitals within a shell are divided into subshells that have the same value of the angular quantum number (l). ◦ lowercase letters are used to indicate different subshells s: l = 0 p: l = 1 d: l = 2 f: l = 3
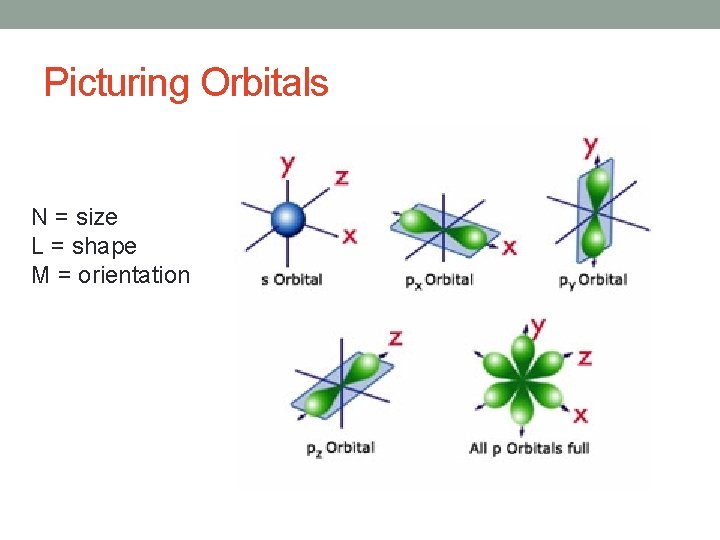
Picturing Orbitals N = size L = shape M = orientation
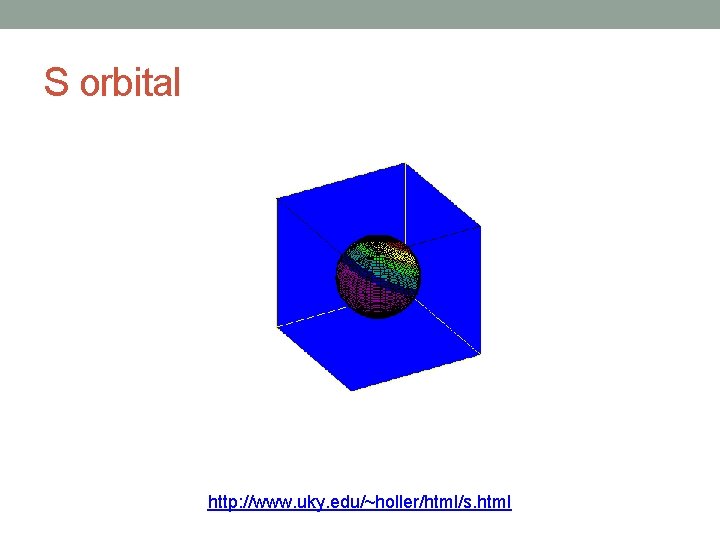
S orbital http: //www. uky. edu/~holler/html/s. html
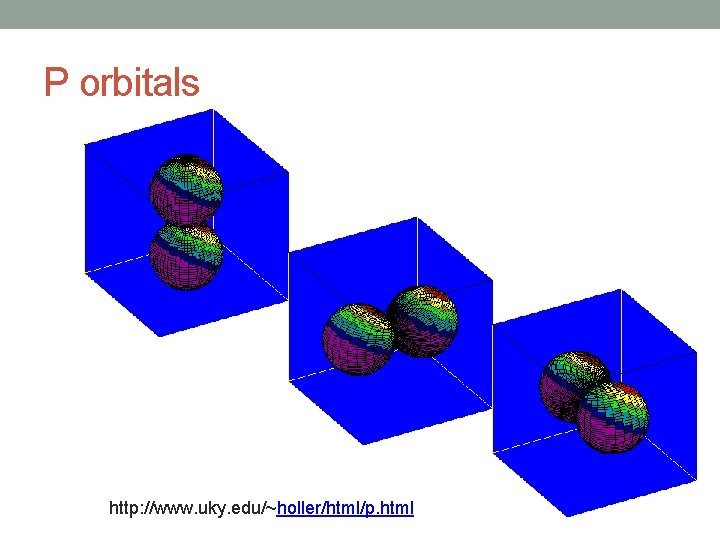
P orbitals http: //www. uky. edu/~holler/html/p. html
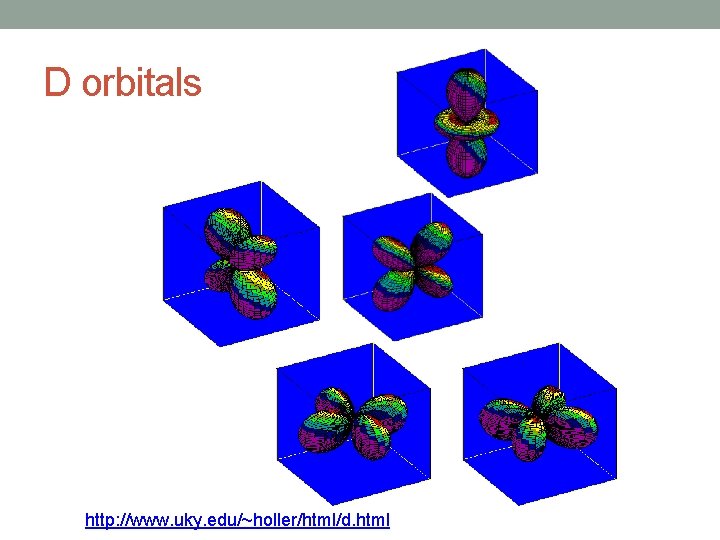
D orbitals http: //www. uky. edu/~holler/html/d. html
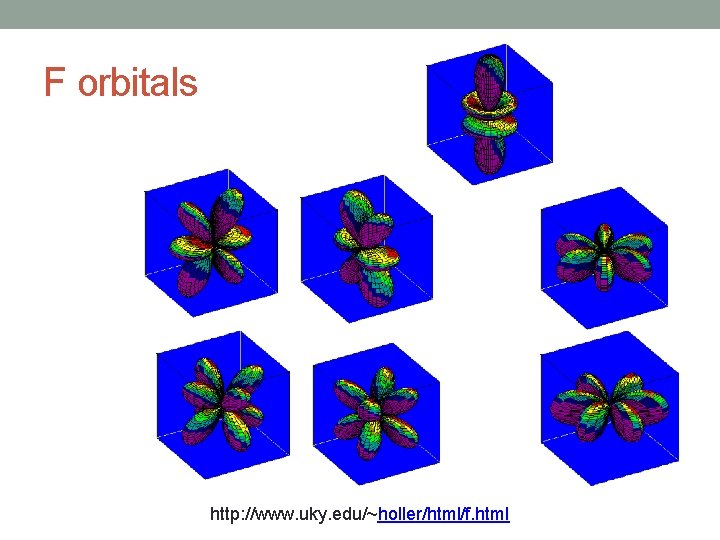
F orbitals http: //www. uky. edu/~holler/html/f. html
• http: //www. youtube. com/watch? v=K- j. Ngq 16 j. EY&feature=related

Break for Hog Hilton Rules 1. Hogs are lazy! They don’t want to go upstairs unless they have to. 2. Hogs are antisocial. They don’t want to room with another hog unless they have to due to Rule 1. 3. Hog stink! You can’t put more than 2 in a room. 4. But hogs still don’t like each other so they will face in the opposite direction in the same room.

Adding Electrons to Orbitals Pauli Exclusion Principle ◦ No two electrons in an atom can have identical quantum numbers The combination of n, l, m, and spin Only 2 electrons per orbital Aufbau Principle Electrons will enter the lowest energy level available Hund’s Rule ◦ Electrons on the same energy level will enter empty orbitals before pairing Electron spin ◦ Electrons in each orbital have opposite spins Spin up or spin down “facing” in opposite directions
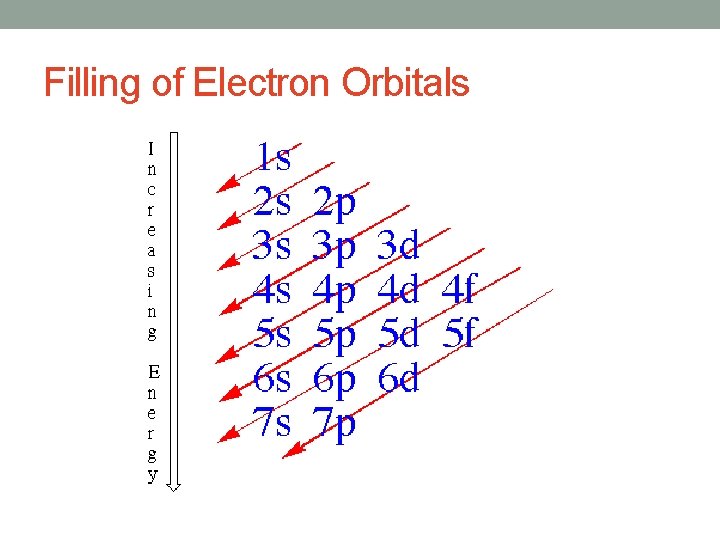
Filling of Electron Orbitals
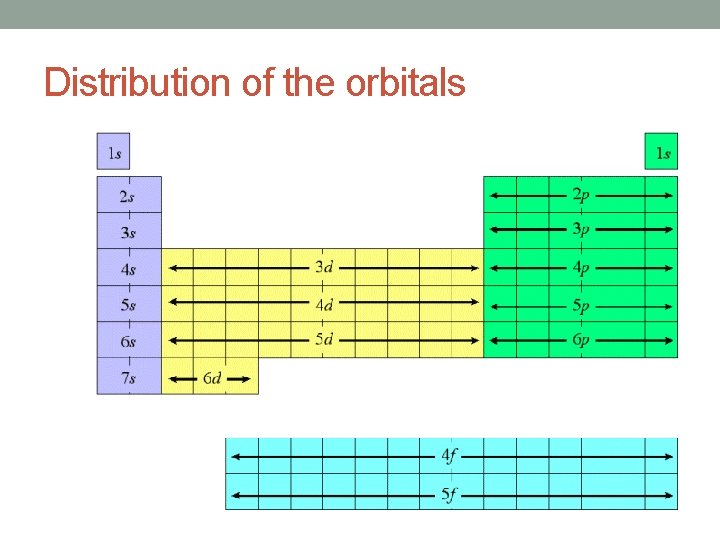
Distribution of the orbitals
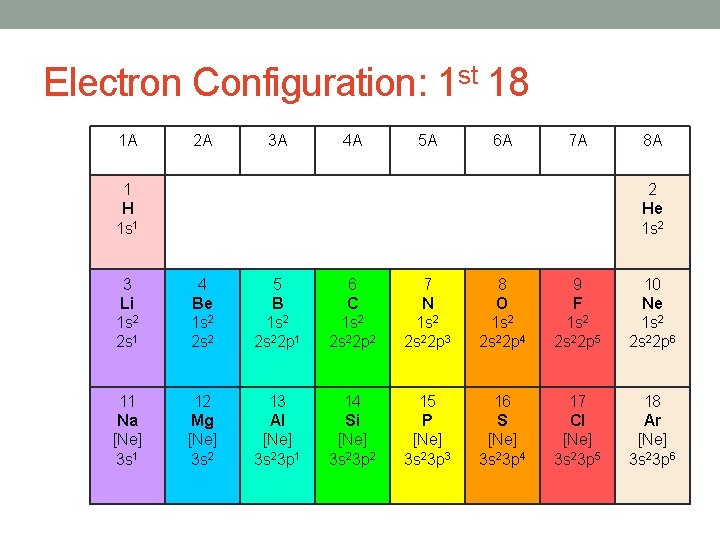
Electron Configuration: 1 st 18 1 A 2 A 3 A 4 A 5 A 6 A 7 A 1 H 1 s 1 8 A 2 He 1 s 2 3 Li 1 s 2 2 s 1 4 Be 1 s 2 2 s 2 5 B 1 s 2 2 s 22 p 1 6 C 1 s 2 2 s 22 p 2 7 N 1 s 2 2 s 22 p 3 8 O 1 s 2 2 s 22 p 4 9 F 1 s 2 2 s 22 p 5 10 Ne 1 s 2 2 s 22 p 6 11 Na [Ne] 3 s 1 12 Mg [Ne] 3 s 2 13 Al [Ne] 3 s 23 p 1 14 Si [Ne] 3 s 23 p 2 15 P [Ne] 3 s 23 p 3 16 S [Ne] 3 s 23 p 4 17 Cl [Ne] 3 s 23 p 5 18 Ar [Ne] 3 s 23 p 6

Standards • 1998: • SC. A. 2. 4. 5 Elements are arranged into groups and families based on similarities in electron structure and their physical and chemical properties can be predicted. • NGSSS • SC. 912. P. 8. 5 Relate properties of atoms and their position in the periodic table to the arrangement of their electrons.
- Slides: 25