Quantum Numbers AP Chemistry Chapter 7 Aufbau Principle

Quantum Numbers AP Chemistry: Chapter 7
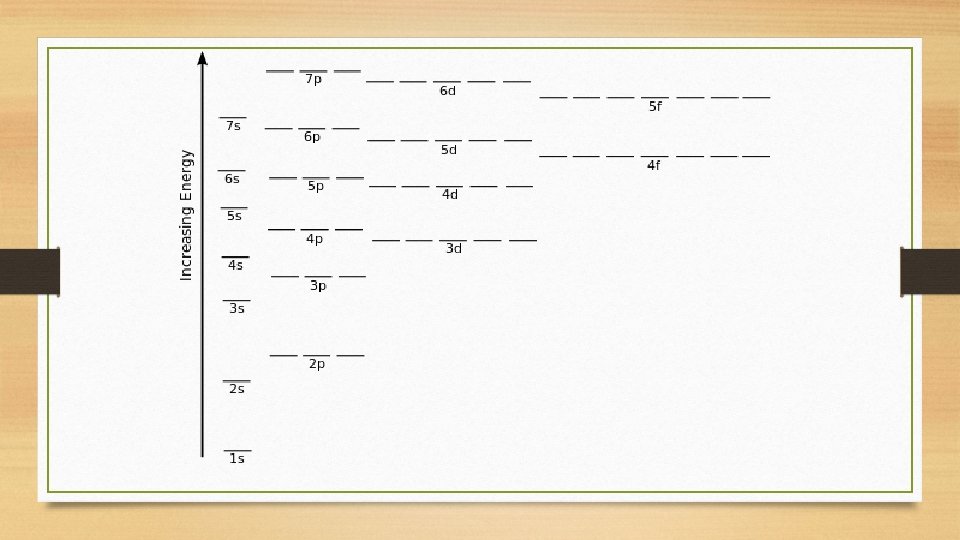
Aufbau Principle • Each electron occupies the lowest energy orbital available. • All of one type of orbital on a certain energy level are the same energy (DEGENERATE) • Due to penetration effect, s orbitals are lower in energy than p orbitals • Electrons in an s orbital spend more time closer to the nucleus, so s orbitals have less energy • d orbitals are higher in energy than the s orbital ONE energy level below • f orbitals are higher in energy than the s orbital TWO energy levels below
Principal Quantum Number (n) • The principal quantum number, n, describes the energy level on which the orbital resides. • The values of n are integers ≥ 1.
Angular Momentum Quantum Number (l) • This quantum number defines the shape of the orbital. • Allowed values of l are integers ranging from 0 to n − 1. • We use letter designations to communicate the different values of l and, therefore, the shapes and types of orbitals.
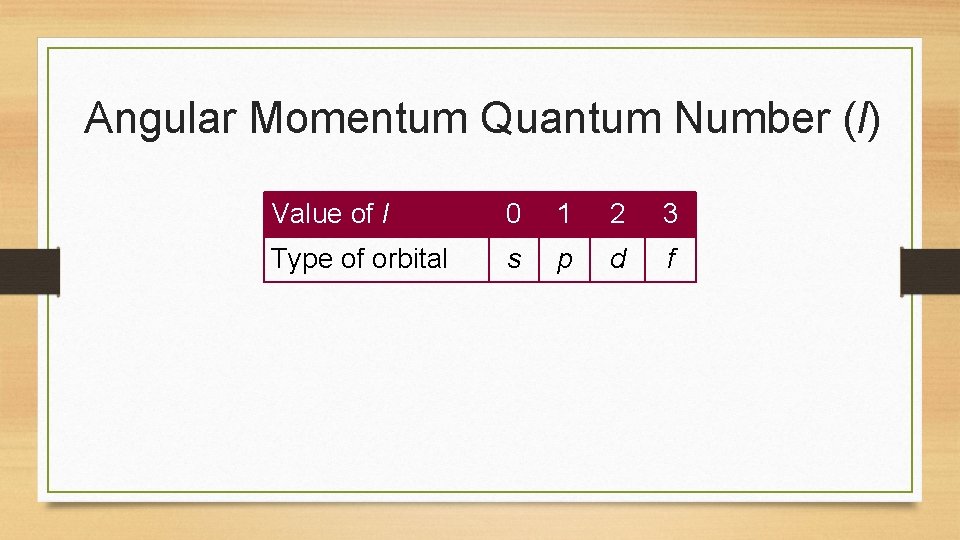
Angular Momentum Quantum Number (l) Value of l 0 1 2 3 Type of orbital s p d f

Magnetic Quantum Number (ml) • The magnetic quantum number describes the threedimensional orientation of the orbital. • Allowed values of ml are integers ranging from −l to l: − l ≤ ml ≤ l • Therefore, on any given energy level, there can be up to 1 s orbital, 3 p orbitals, 5 d orbitals, 7 f orbitals, and so forth.
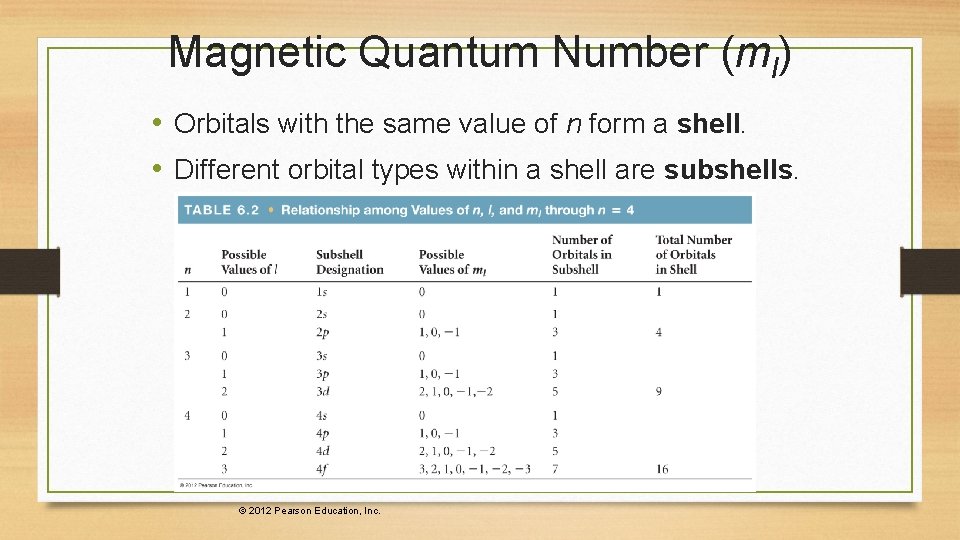
Magnetic Quantum Number (ml) • Orbitals with the same value of n form a shell. • Different orbital types within a shell are subshells. © 2012 Pearson Education, Inc.
Electron Spin • Experiments by Stern and Gerlach showed that a beam of silver atoms is split in two by a magnetic field. • The experiment reveals that the electrons spin on their axis. • As they spin, they generate a magnetic field. • Spinning charged particles generates a magnetic field. • If there is an even number of electrons, about half the atoms will have a net magnetic field pointing “north” and the other half will have a net magnetic field pointing “south. ”

Pauli Exclusion Principle • No two electrons in an atom may have the same set of four quantum numbers. • Therefore, no orbital may have more than two electrons, and they must have opposite spins.
The Property of Electron Spin • Spin is a fundamental property of all electrons. • All electrons have the same amount of spin. • The orientation of the electron spin is quantized, it can only be in one direction or its opposite. • Spin up or spin down • The electron’s spin adds a fourth quantum number to the description of electrons in an atom, called the spin quantum number, ms
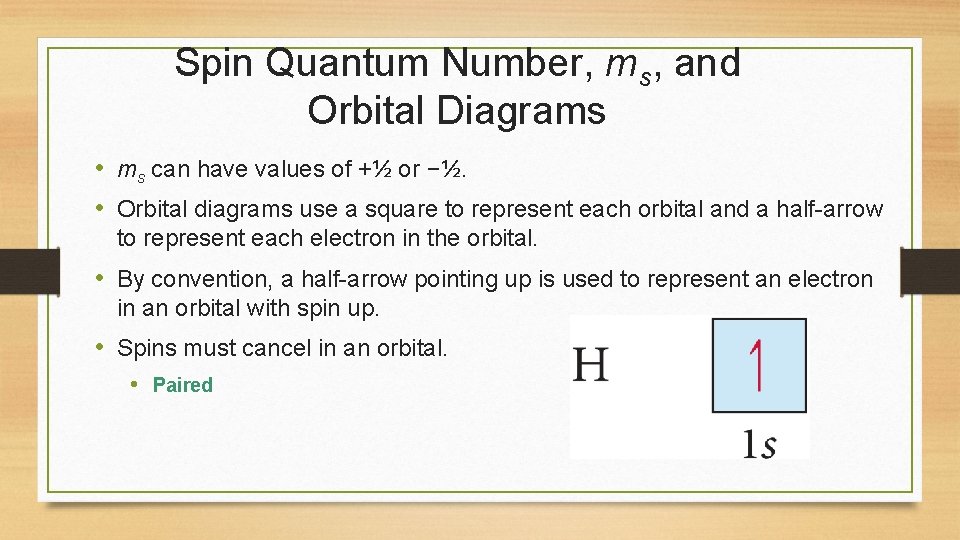
Spin Quantum Number, ms, and Orbital Diagrams • ms can have values of +½ or −½. • Orbital diagrams use a square to represent each orbital and a half-arrow to represent each electron in the orbital. • By convention, a half-arrow pointing up is used to represent an electron in an orbital with spin up. • Spins must cancel in an orbital. • Paired
Hund’s Rule • The lowest energy configuration for an atom is the one having the maximum number of unpaired electrons allowed by the Pauli principle in a particular set of degenerate (same energy) orbitals. • In a set of degenerate orbitals, fill one electron at a time before pairing in same orbital 13
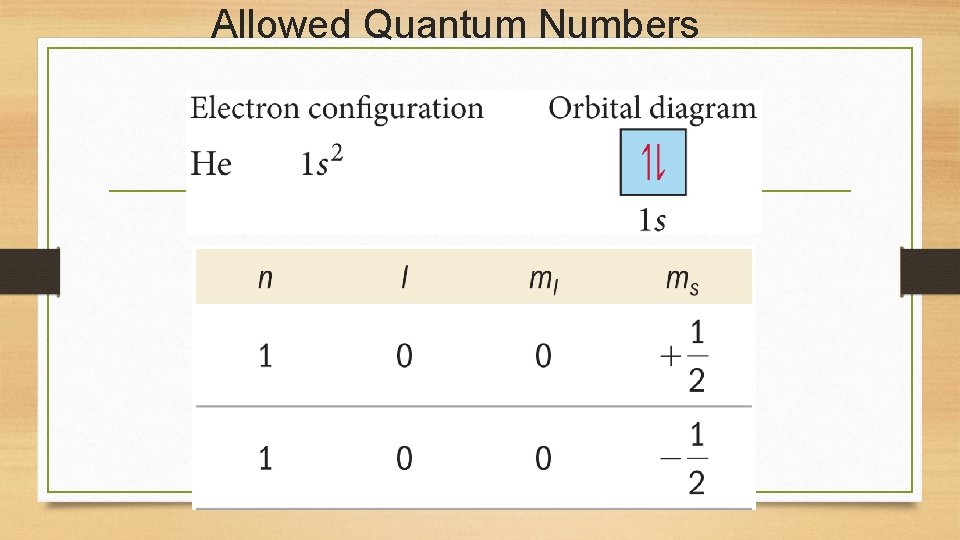
Allowed Quantum Numbers
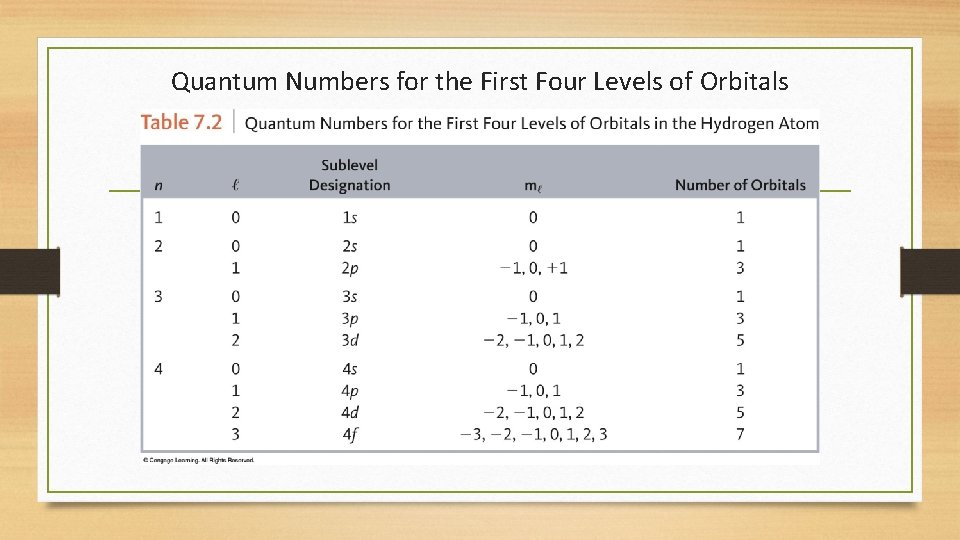
Quantum Numbers for the First Four Levels of Orbitals
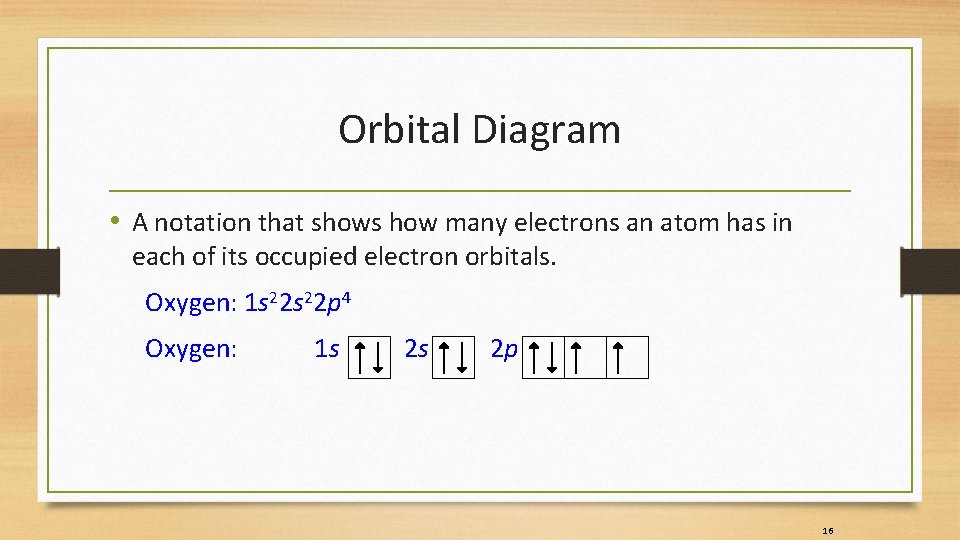
Orbital Diagram • A notation that shows how many electrons an atom has in each of its occupied electron orbitals. Oxygen: 1 s 22 p 4 Oxygen: 1 s 2 s 2 p 16
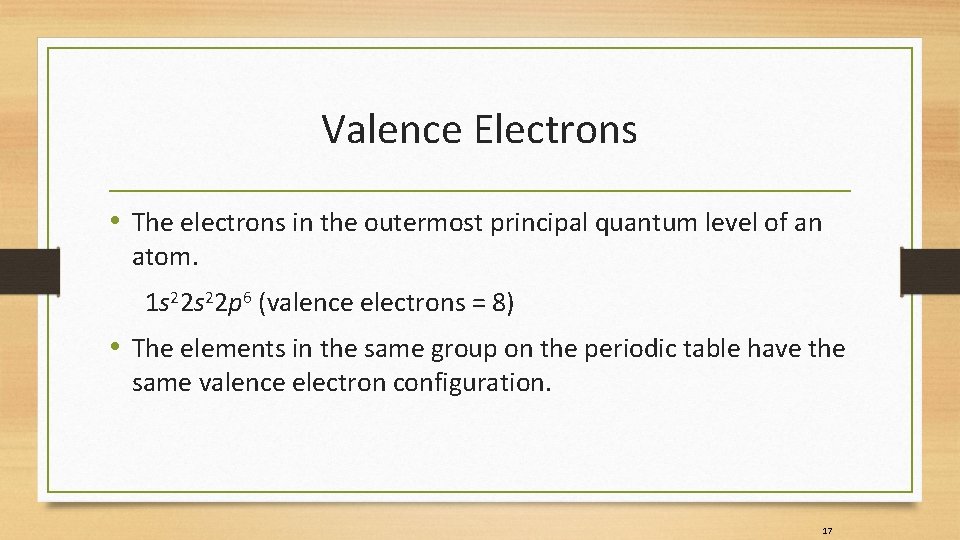
Valence Electrons • The electrons in the outermost principal quantum level of an atom. 1 s 22 p 6 (valence electrons = 8) • The elements in the same group on the periodic table have the same valence electron configuration. 17
- Slides: 17