QUANTUM NUMBERS a b c d Set of
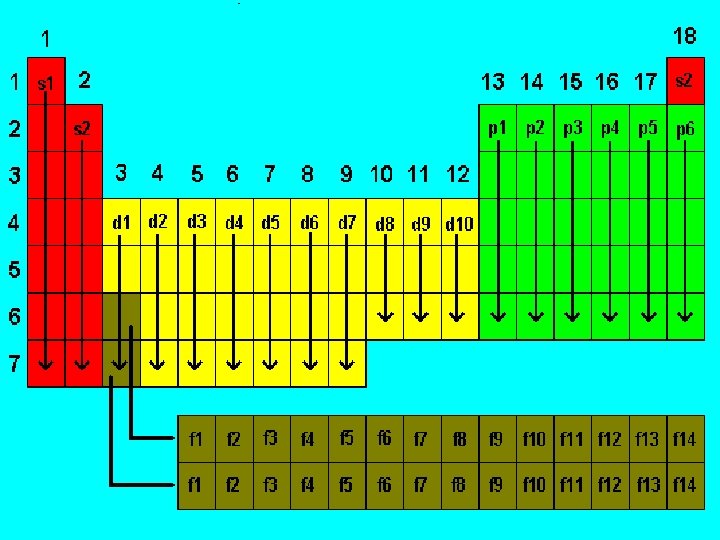

QUANTUM NUMBERS a. b. c. d. Set of 4 numbers used to describe the electrons in terms of : Distance from the nucleus Shape of the orbitals Orientation in space Direction of electron spin

PRINCIPAL QUANTUM NUMBER, n - - Refers to the main energy levels Related to the average distance of the electron from the nucleus Can only have integral values of n = 1, 2, 3, 4 etc.
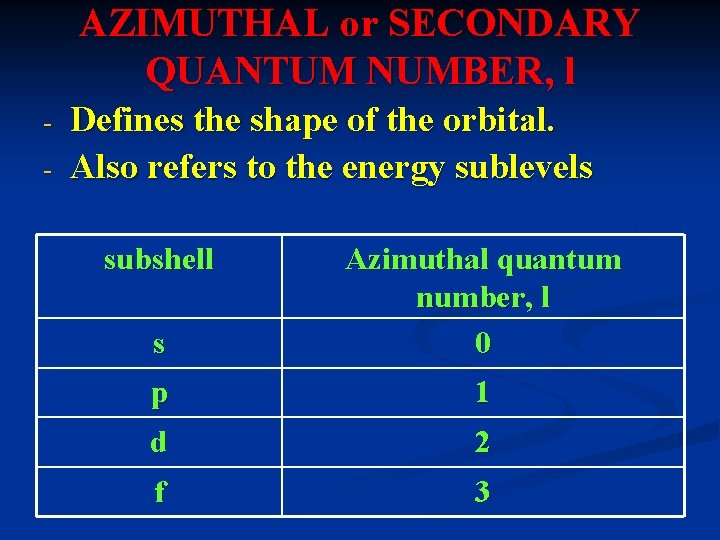
AZIMUTHAL or SECONDARY QUANTUM NUMBER, l - Defines the shape of the orbital. Also refers to the energy sublevels subshell s Azimuthal quantum number, l 0 p 1 d 2 f 3
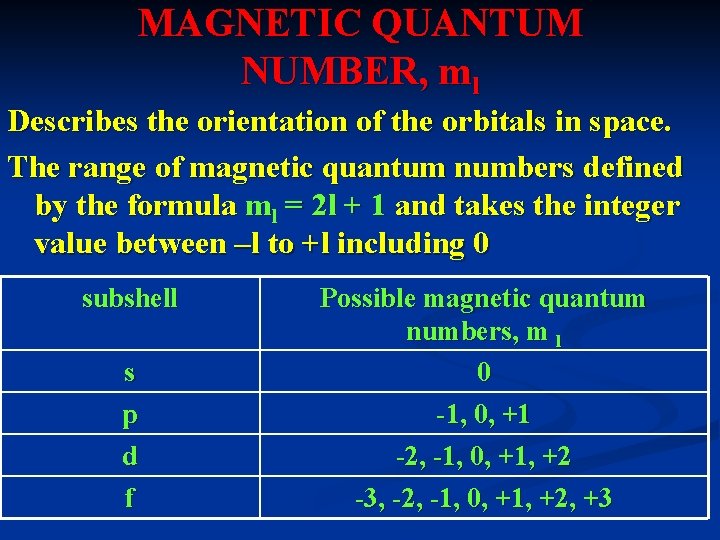
MAGNETIC QUANTUM NUMBER, ml Describes the orientation of the orbitals in space. The range of magnetic quantum numbers defined by the formula ml = 2 l + 1 and takes the integer value between –l to +l including 0 subshell s p d f Possible magnetic quantum numbers, m l 0 -1, 0, +1 -2, -1, 0, +1, +2 -3, -2, -1, 0, +1, +2, +3

SPIN QUANTUM NUMBER, ms - differentiates how two electrons behave under a magnetic field. - Can only have two possible values +½ and -½

Example. What are the possible quantum numbers for the outermost electron of oxygen? SOLUTION: Identify the final orbital occupied by the electron. 1 s 2 2 p 4 2 p 4 n= 2 l= 1 ml = ms = -1, 0, +1 +½ , -½

What are the possible quantum numbers for the outermost electron of chromium? SOLUTION: Identify the final orbital occupied by the electron. 1 s 2 2 p 6 3 s 2 3 p 6 4 s 1 3 d 5 3 d 5 n= 3 l= 2 ml = ms = -2, -1, 0, +1, +2 +½ , -½

For each subshells given below, give the maximum number of orbitals and electrons possible Subshell or sublevel 8 s 3 p 3 d 6 f 6 s 5 d 4 f Number of orbitals Number of electrons

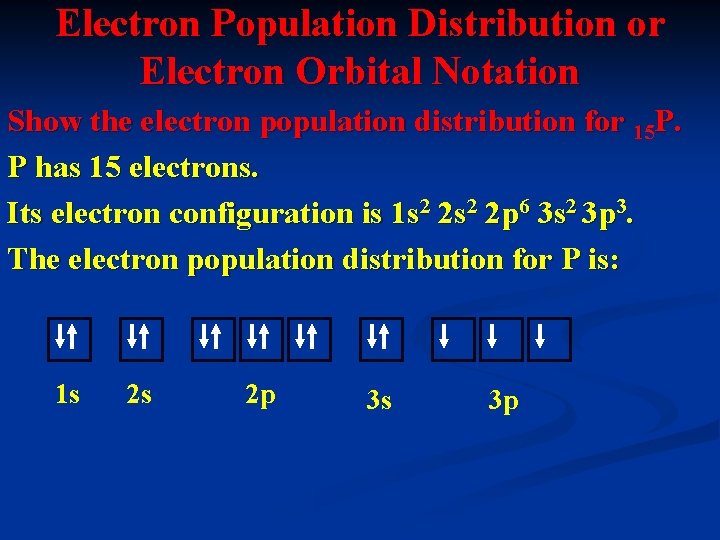
Electron Population Distribution or Electron Orbital Notation Show the electron population distribution for 15 P. P has 15 electrons. Its electron configuration is 1 s 2 2 p 6 3 s 2 3 p 3. The electron population distribution for P is: 1 s 2 s 2 p 3 s 3 p
- Slides: 11