Quantum Number Number that specifies the properties of

Quantum Number � Number that specifies the properties of the atomic orbitals � Tells us the distance from the nucleus and the shape of the orbital

Electron Configuration
Principal Quantum Number � Main level or shell � These are the Bohr energy levels n = 1, n = 2, n = 3 � As n increases, the distance from the nucleus increases
Sublevel � Each main level is divided into sublevels � Four types of sublevels s p d f
Orbital � Each sublevel is made of orbitals � Every orbital can hold 2 electrons

� � s – 1 orbital – 2 electrons p – 3 orbitals – 6 electrons d – 5 orbitals – 10 electrons f – 7 orbitals – 14 electrons
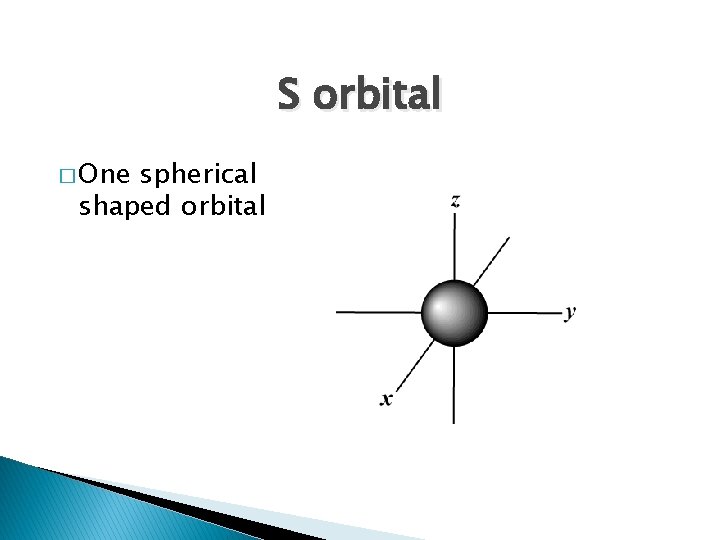
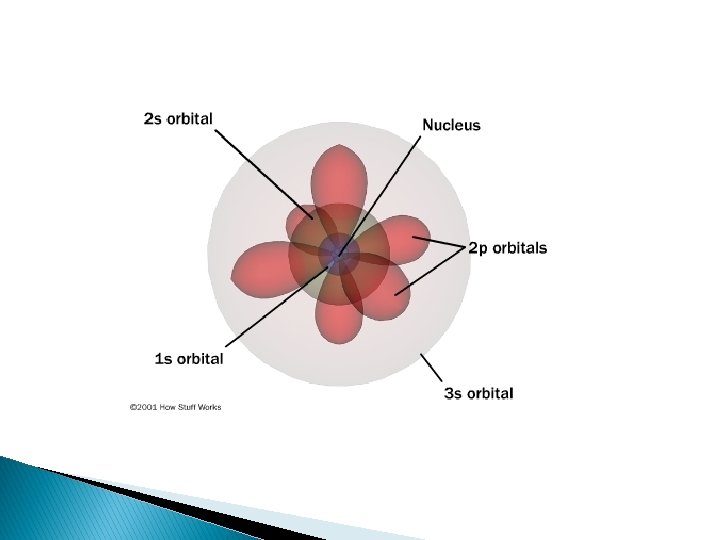
S orbital � One spherical shaped orbital
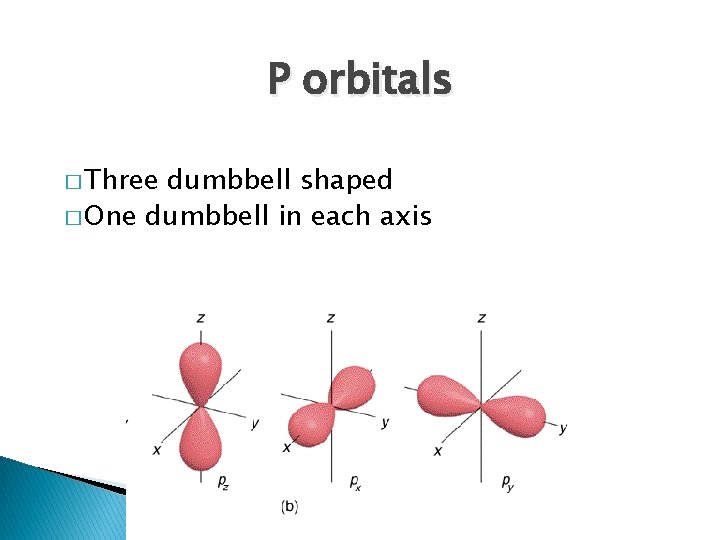
P orbitals � Three dumbbell shaped � One dumbbell in each axis
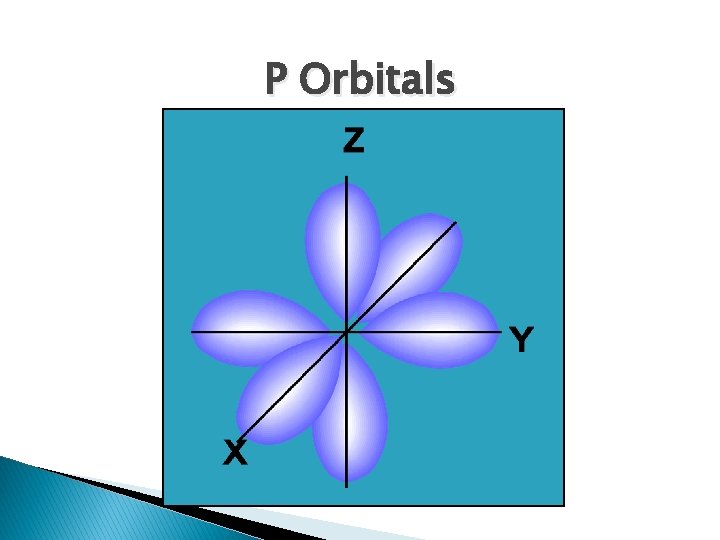
P Orbitals
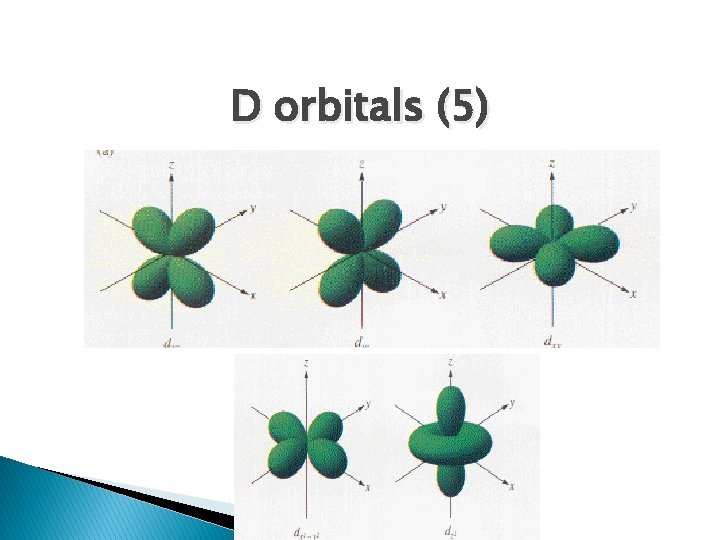
D orbitals (5)
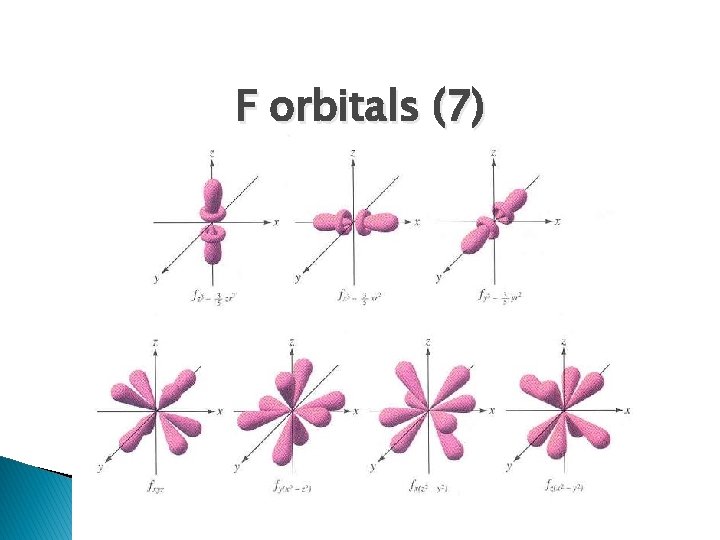
F orbitals (7)
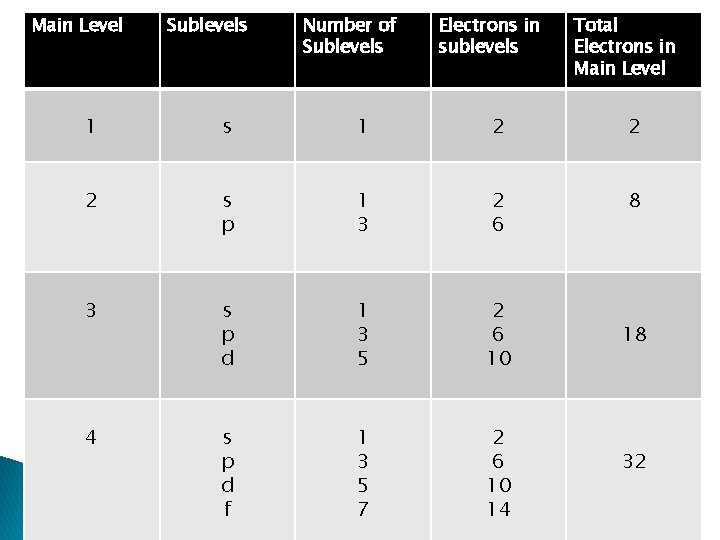
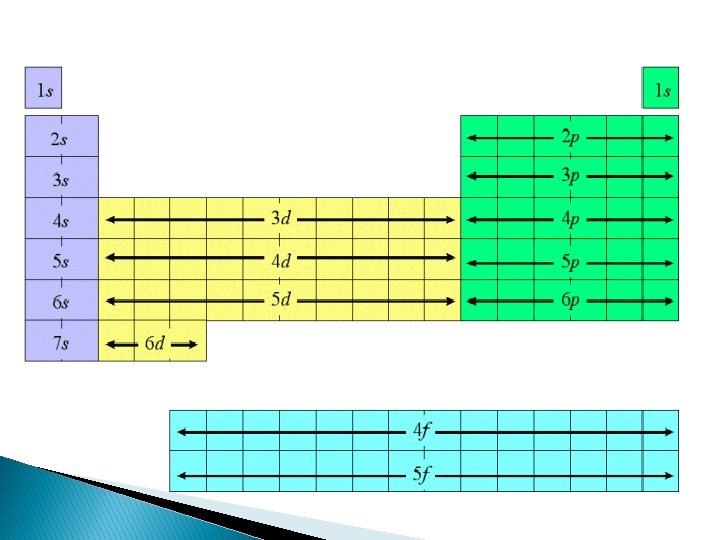
Main Level Sublevels Number of Sublevels Electrons in sublevels Total Electrons in Main Level 1 s 1 2 2 2 s p 1 3 2 6 8 3 s p d 1 3 5 2 6 10 4 s p d f 1 3 5 7 2 6 10 14 18 32
Electron Configuration � Arrangement of electrons in an atom � Aufbau Principle – electrons fill into an atom starting with the lowest energy levels
Electron Spin � Way which the electrons rotate on their axis � Pauli Exclusion Principle – in order for two electrons to occupy the same orbital, they must have opposite spin
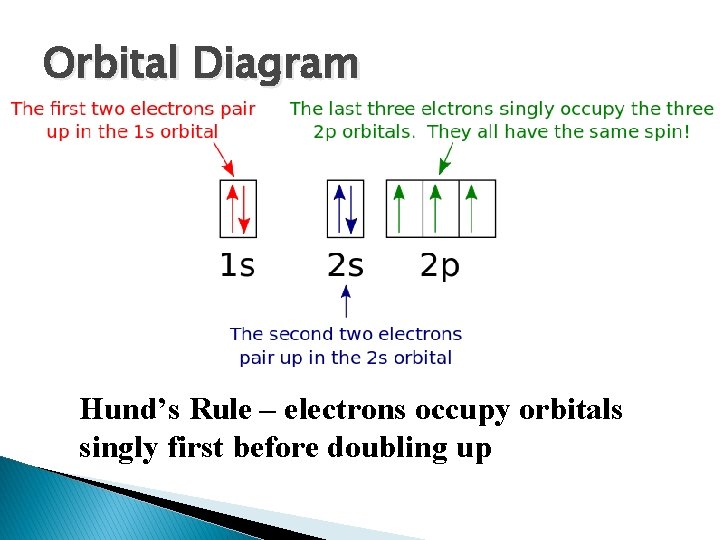
Orbital Diagram Hund’s Rule – electrons occupy orbitals singly first before doubling up
Writing Configurations � Write the configuration for each of the below C S Br Na Cl Kr

Valence Electrons � Electrons in the last main energy level � These are the electrons farthest out on the atom � These will interact with other atoms � These are the electrons involved in chemical reactions � There a maximum of 8 valence electrons

How to find valence e� Write configuration and count electrons in last main energy level � Examples: Find valence electrons for C Na P Fe Ar

Octet Rule � Atoms will give up, accept, or share electrons in order to achieve a filled valence shell (8 valence electrons)

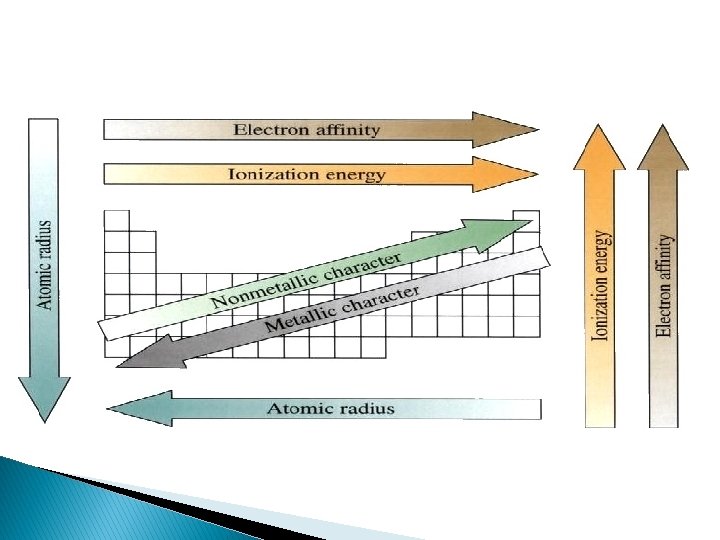
Metallic Character � Metals become more reactive (more metallic in character) as you go down a group � Most metallic elements bottom left corner of the periodic table � Least metallic, top right corner
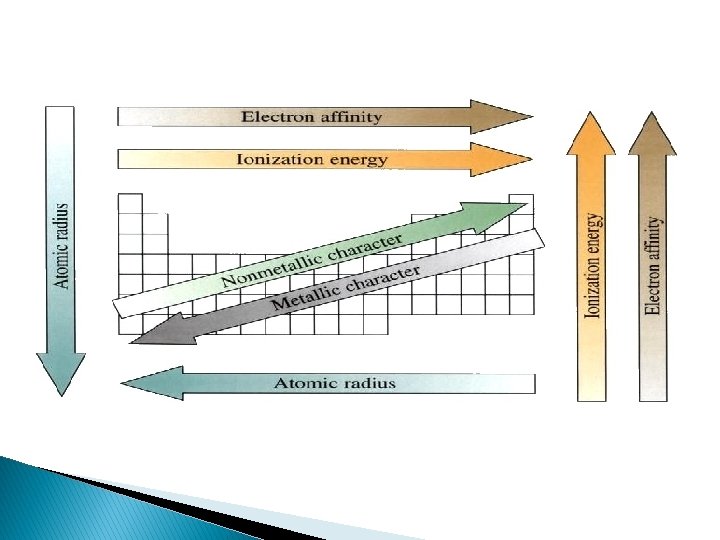


Ionization Energy � Energy required to remove the most loosely held electron from an atom � The greater the ionization energy, the more strongly the atom holds onto its electrons � M + energy → M+ + e � Ionization energy increases as moving across a period � Ionization energy decreases as moving down a group
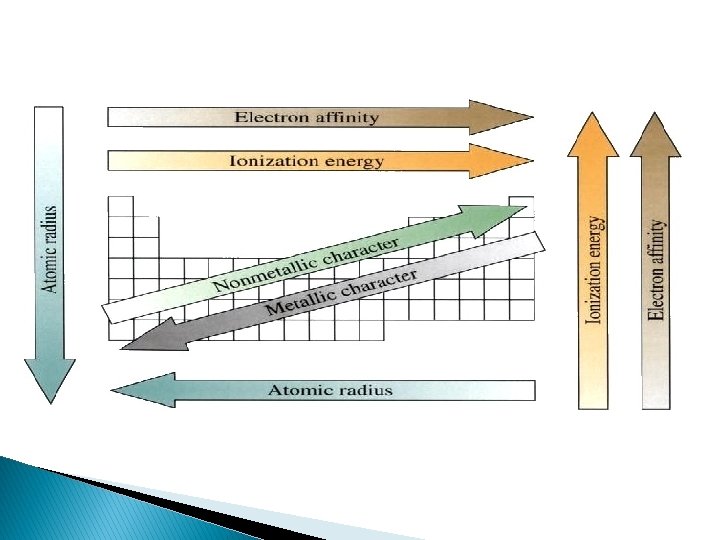
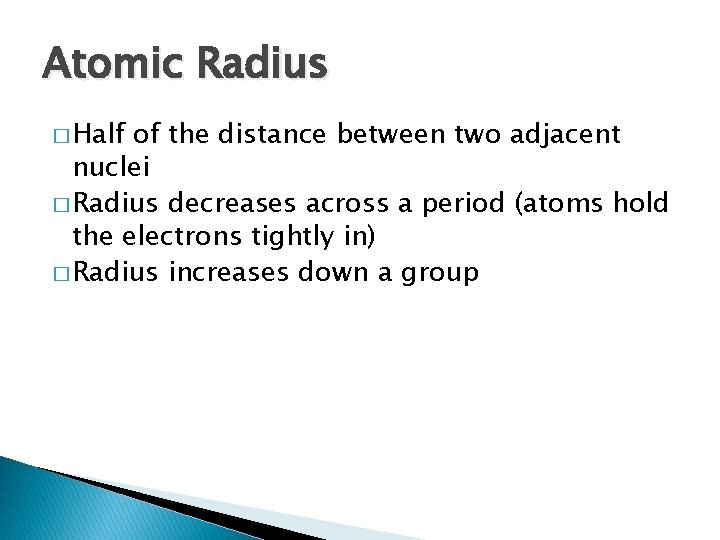
Atomic Radius � Half of the distance between two adjacent nuclei � Radius decreases across a period (atoms hold the electrons tightly in) � Radius increases down a group
- Slides: 28