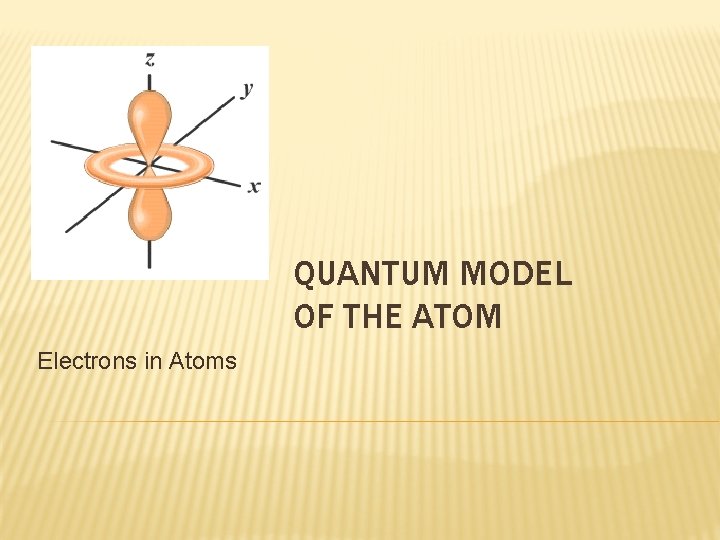
QUANTUM MODEL OF THE ATOM Electrons in Atoms
![STABILITY Electron Configuration Exceptions y. Copper EXPECT: [Ar] 4 s 2 3 d 9 STABILITY Electron Configuration Exceptions y. Copper EXPECT: [Ar] 4 s 2 3 d 9](https://slidetodoc.com/presentation_image_h2/09cf7525b9b8f14619d6e6a70b5e1d1c/image-8.jpg)
![STABILITY Electron Configuration Exceptions y. Chromium EXPECT: [Ar] 4 s 2 3 d 4 STABILITY Electron Configuration Exceptions y. Chromium EXPECT: [Ar] 4 s 2 3 d 4](https://slidetodoc.com/presentation_image_h2/09cf7525b9b8f14619d6e6a70b5e1d1c/image-9.jpg)





- Slides: 32

QUANTUM MODEL OF THE ATOM Electrons in Atoms

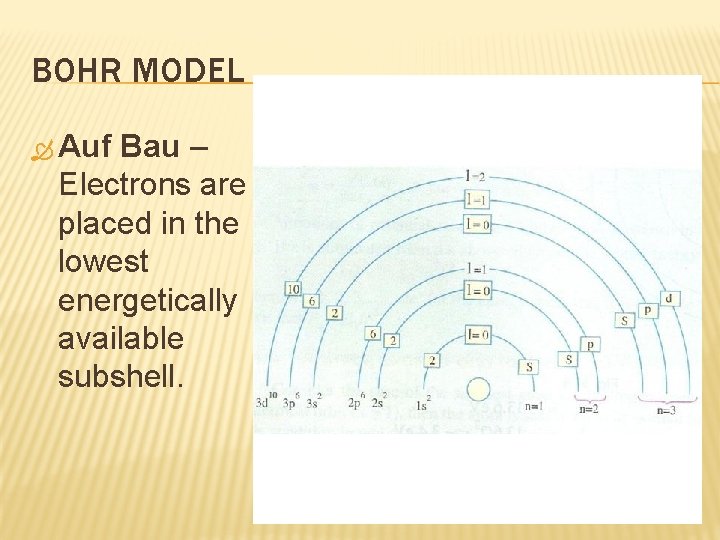
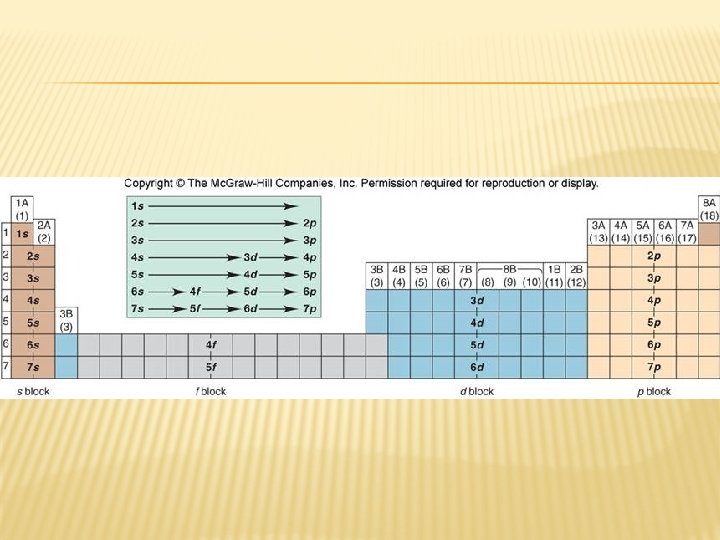
BOHR MODEL Auf Bau – Electrons are placed in the lowest energetically available subshell.

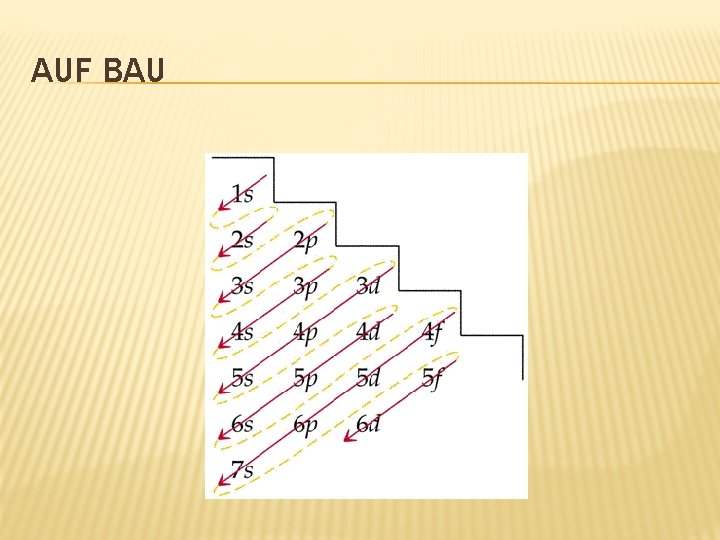
AUF BAU

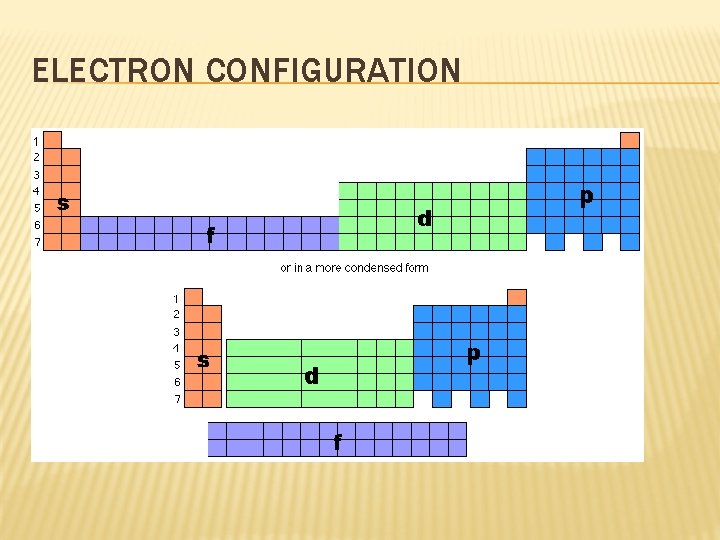
ELECTRON CONFIGURATION


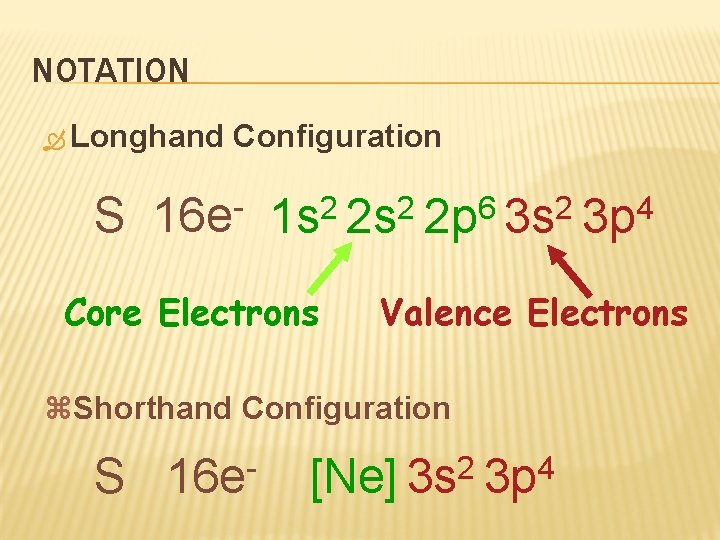
NOTATION Longhand S Configuration 16 e 6 2 2 2 1 s 2 s 2 p 3 s Core Electrons Valence Electrons z. Shorthand Configuration S 16 e 4 3 p 2 4 [Ne] 3 s 3 p

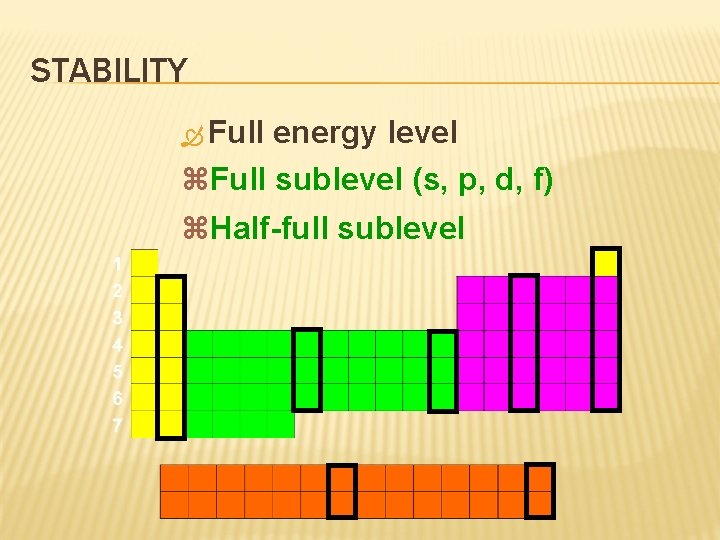
STABILITY Full energy level z. Full sublevel (s, p, d, f) z. Half-full sublevel
![STABILITY Electron Configuration Exceptions y Copper EXPECT Ar 4 s 2 3 d 9 STABILITY Electron Configuration Exceptions y. Copper EXPECT: [Ar] 4 s 2 3 d 9](https://slidetodoc.com/presentation_image_h2/09cf7525b9b8f14619d6e6a70b5e1d1c/image-8.jpg)
STABILITY Electron Configuration Exceptions y. Copper EXPECT: [Ar] 4 s 2 3 d 9 ACTUALLY: [Ar] 4 s 1 3 d 10 y. Copper gains stability with a full d-sublevel.
![STABILITY Electron Configuration Exceptions y Chromium EXPECT Ar 4 s 2 3 d 4 STABILITY Electron Configuration Exceptions y. Chromium EXPECT: [Ar] 4 s 2 3 d 4](https://slidetodoc.com/presentation_image_h2/09cf7525b9b8f14619d6e6a70b5e1d1c/image-9.jpg)
STABILITY Electron Configuration Exceptions y. Chromium EXPECT: [Ar] 4 s 2 3 d 4 ACTUALLY: [Ar] 4 s 1 3 d 5 y. Chromium gains stability with a half-full d-sublevel.

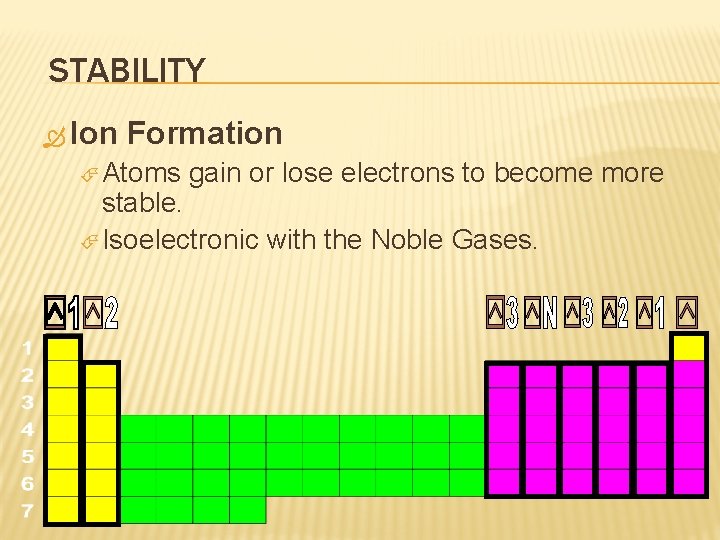
STABILITY Ion Formation Atoms gain or lose electrons to become more stable. Isoelectronic with the Noble Gases.

STABILITY Ion Electron Configuration Write EX: 2 O the e- config for the closest Noble Gas Oxygen ion O 2 - Ne 10 e [He] 2 2 s 6 2 p

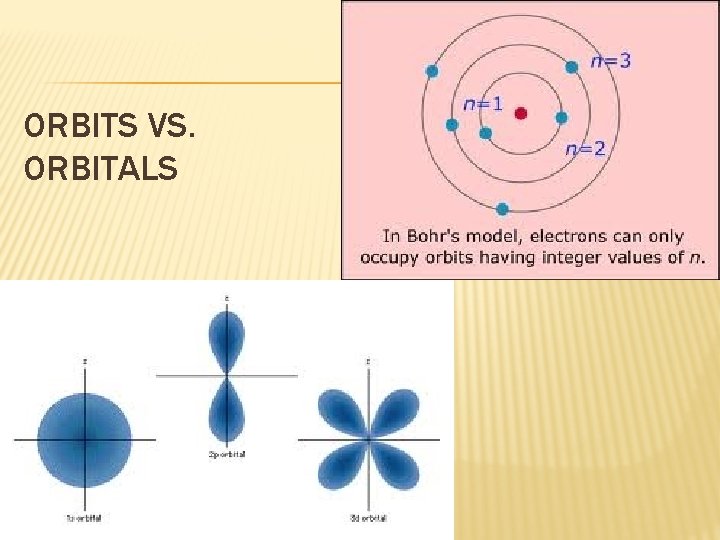
ORBITS VS. ORBITALS

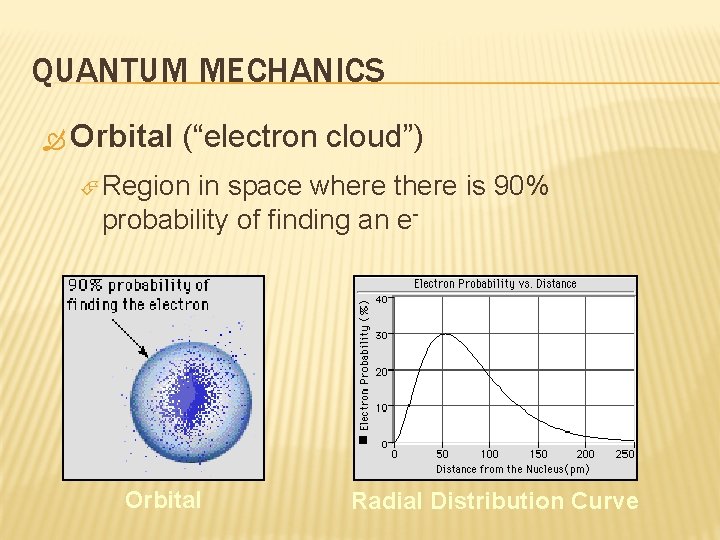
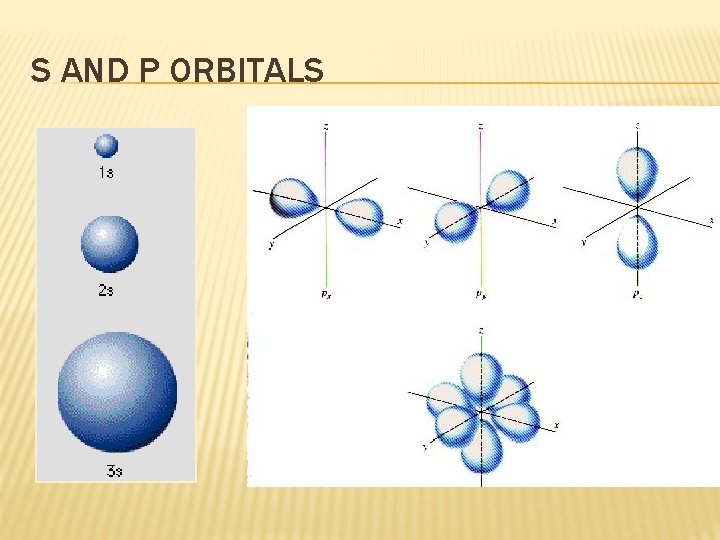
QUANTUM MECHANICS Orbital (“electron cloud”) Region in space where there is 90% probability of finding an e- Orbital Radial Distribution Curve

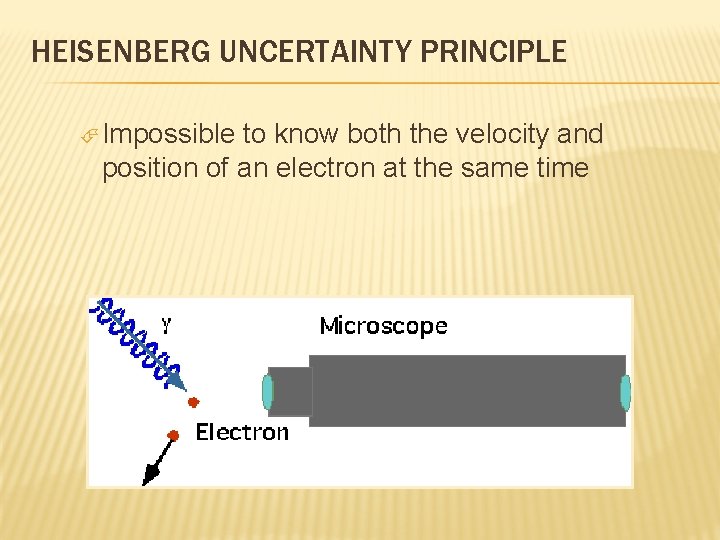
HEISENBERG UNCERTAINTY PRINCIPLE Impossible to know both the velocity and position of an electron at the same time

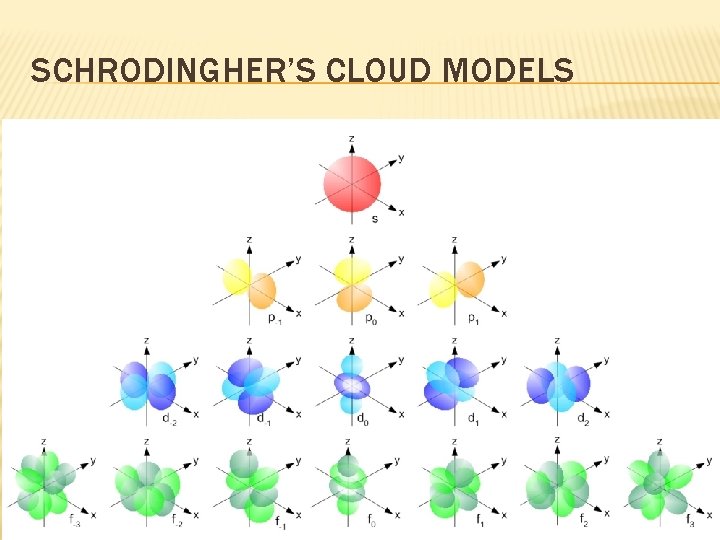
SCHRODINGHER’S CLOUD MODELS

S AND P ORBITALS

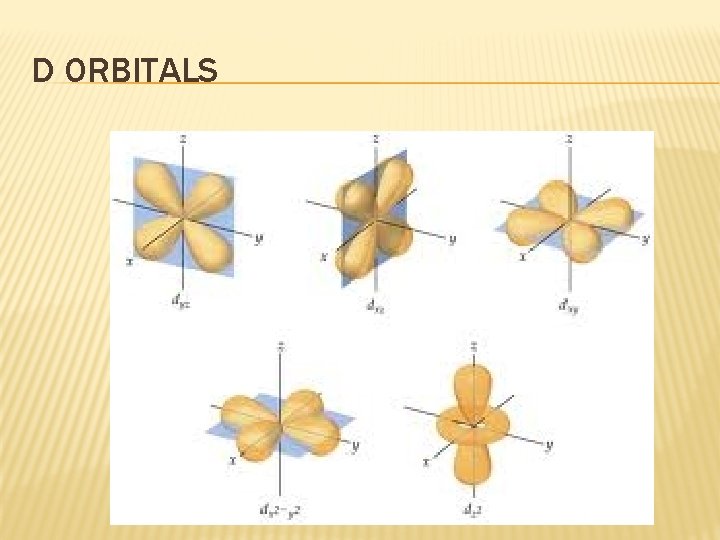
D ORBITALS

QUANTUM NUMBERS Four Quantum Numbers: Specify the “address” of each electron in an atom UPPER LEVEL

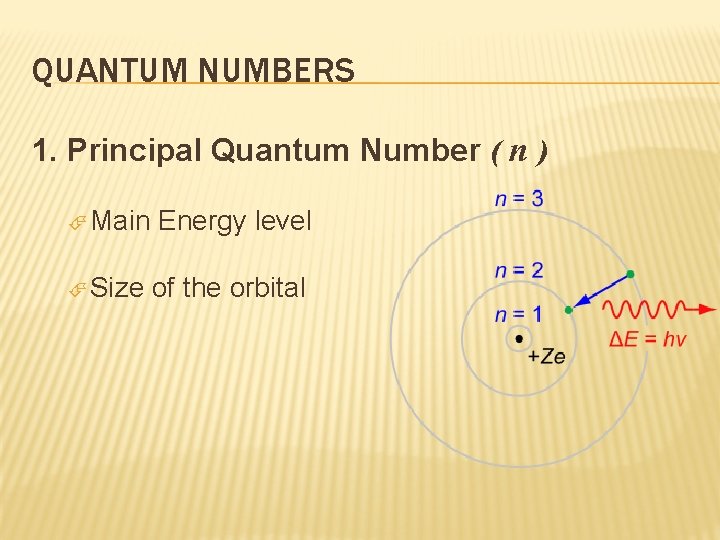
QUANTUM NUMBERS 1. Principal Quantum Number ( n ) Main Size Energy level of the orbital

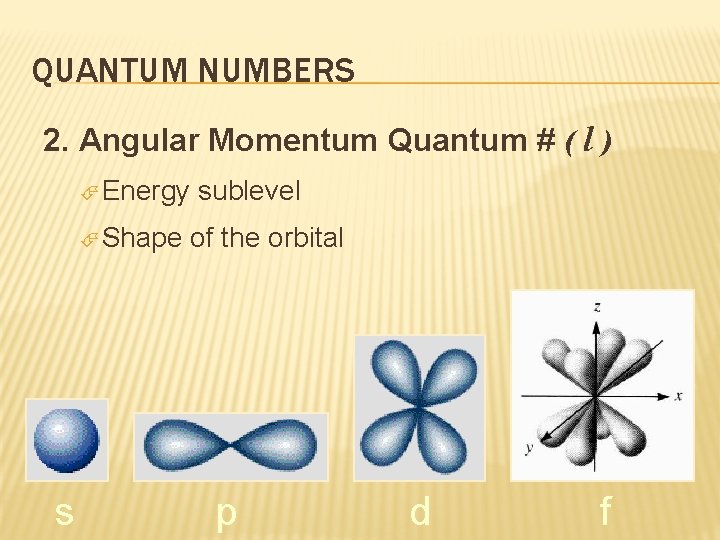
QUANTUM NUMBERS 2. Angular Momentum Quantum # ( l ) Energy Shape s sublevel of the orbital p d f

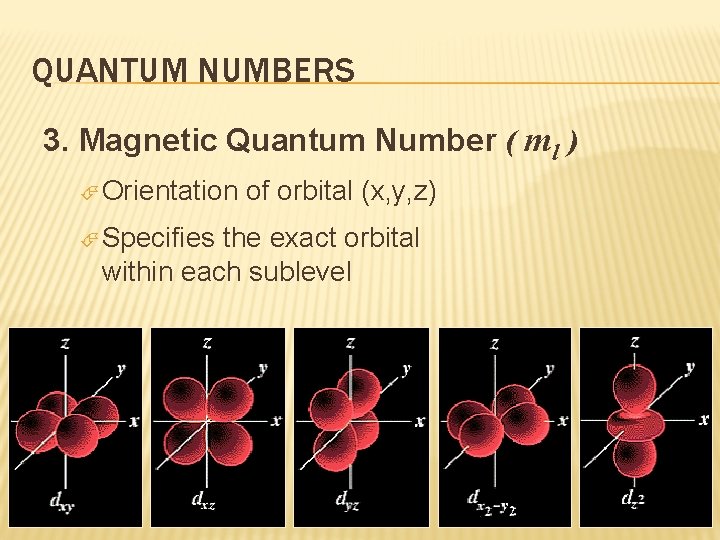
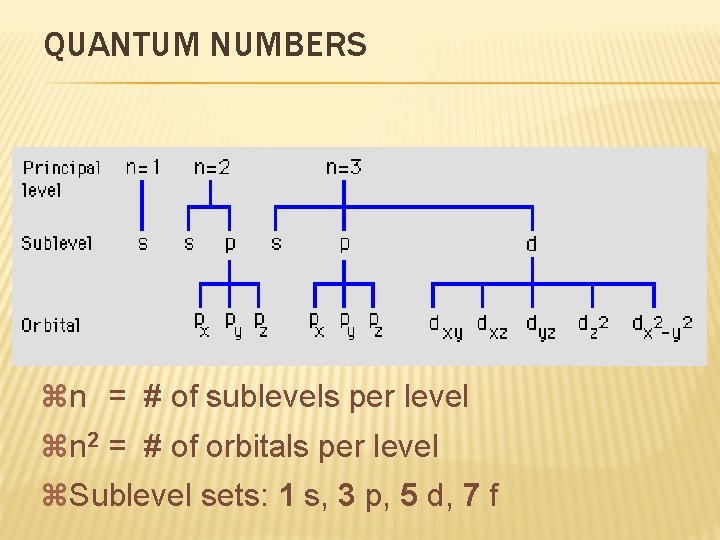
QUANTUM NUMBERS 3. Magnetic Quantum Number ( ml ) Orientation Specifies of orbital (x, y, z) the exact orbital within each sublevel

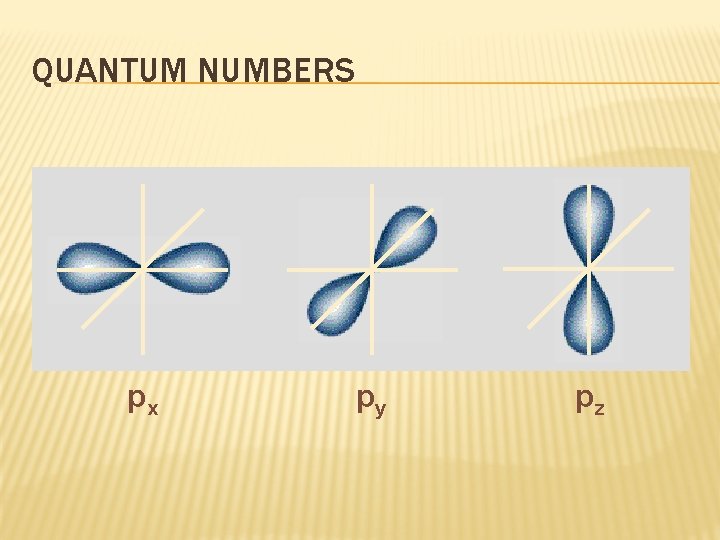
QUANTUM NUMBERS px py pz

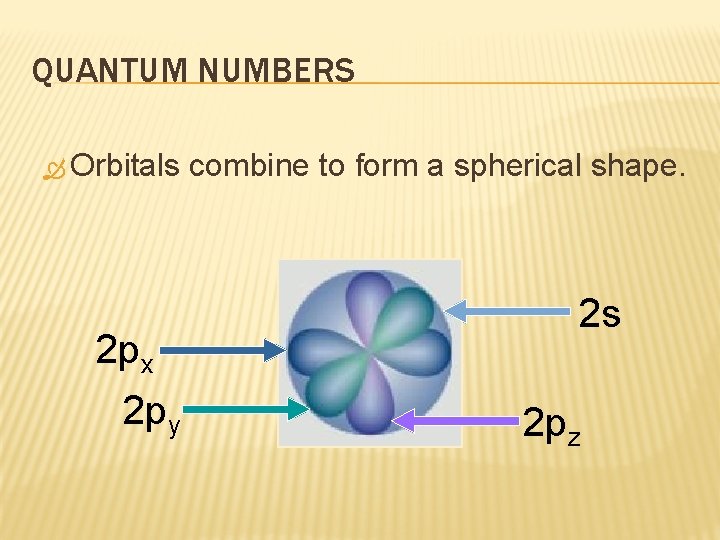
QUANTUM NUMBERS Orbitals 2 px 2 py combine to form a spherical shape. 2 s 2 pz

QUANTUM NUMBERS zn = # of sublevels per level zn 2 = # of orbitals per level z. Sublevel sets: 1 s, 3 p, 5 d, 7 f

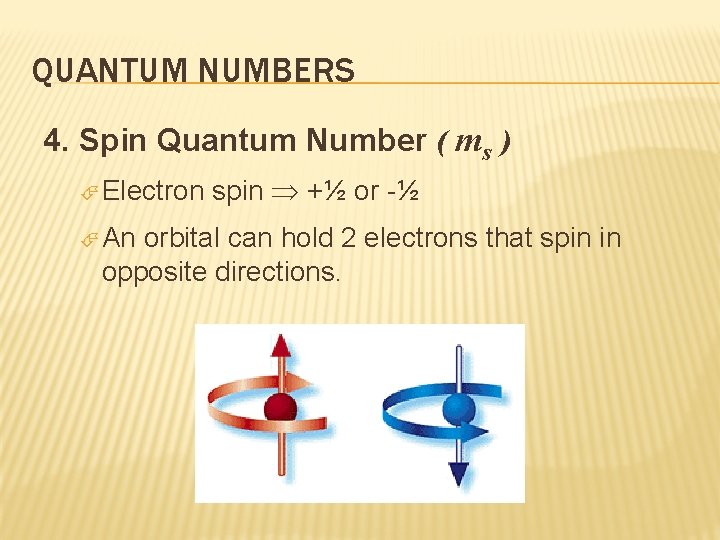
QUANTUM NUMBERS 4. Spin Quantum Number ( ms ) Electron An spin +½ or -½ orbital can hold 2 electrons that spin in opposite directions.

QUANTUM NUMBERS Pauli Exclusion Principle No two electrons in an atom can have the same 4 quantum numbers. Each e- has a unique “address”: 1. Principal # 2. Ang. Mom. # 3. Magnetic # 4. Spin # energy level sublevel (s, p, d, f) Orbital (X, Y, Z) Electron (+1/2, -1/2)

PAULI EXCLUSION PRINCIPLE Each orbital can hold TWO electrons with opposite spins.

HUND’S RULE Orbitals of equal energy must each possess one electron before any can possess a second. “Empty Bus Seat Rule” WRONG RIGHT

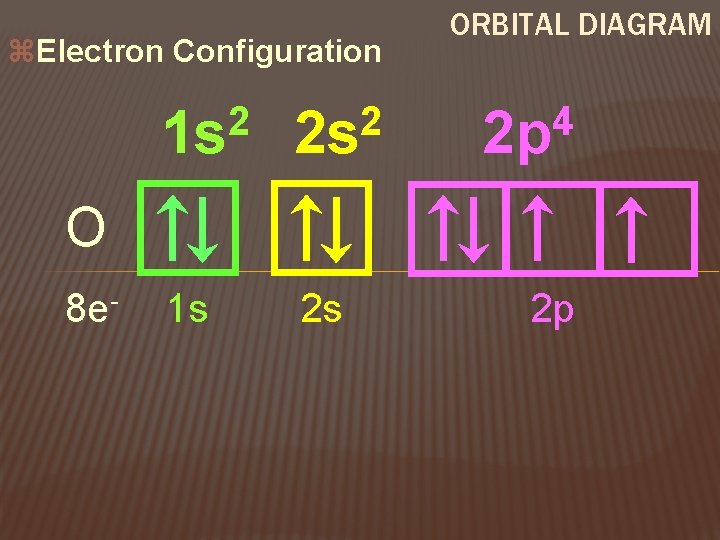
z. Electron Configuration 2 1 s 2 2 s 1 s 2 s ORBITAL DIAGRAM 4 2 p O 8 e- 2 p

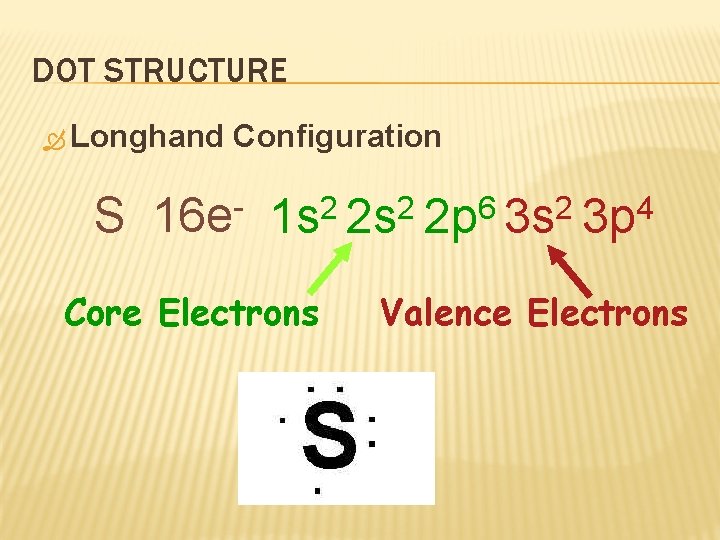
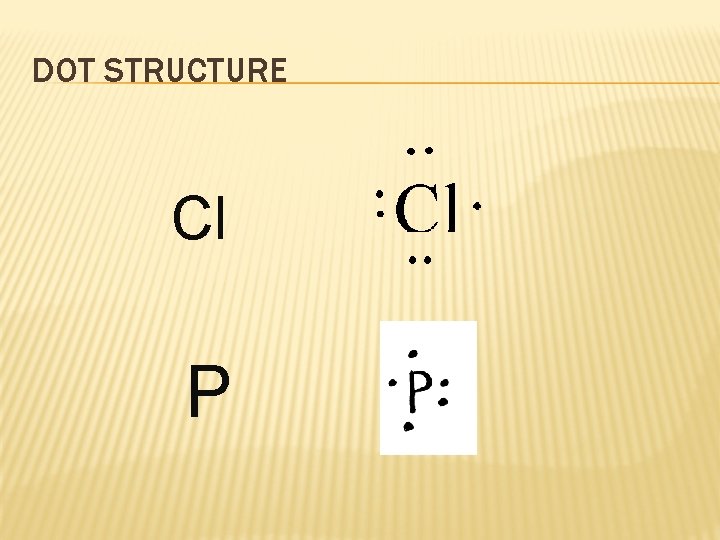
DOT STRUCTURE Longhand S Configuration 16 e 6 2 2 2 1 s 2 s 2 p 3 s Core Electrons 4 3 p Valence Electrons

DOT STRUCTURE Cl P

FEELING OVERWHELMED? Read Chapter 10