Quantum Model of the Atom and Quantum Numbers
Quantum Model of the Atom and Quantum Numbers
Bohr's Model of an Atom • Bohr proposed a model of an atom based on Hydrogen atom (H has 1 electron!!!) He pictured electrons in orbit around a central nucleus Evidence for his work was obtained from emission line spectra for hydrogen atom Bohr's model imposed certain restrictions
Bohr's Model The atom has only specific, allowable energy levels, called stationary states. Each stationary state corresponds to the atom’s electrons occupying fixed, circular orbits around the nucleus.
Bohr's Model – While in one of its stationary states, atoms do not emit energy – An atom changes stationary states by emitting or absorbing a specific quantity of energy that is exactly equal to the difference in energy between two stationary states.
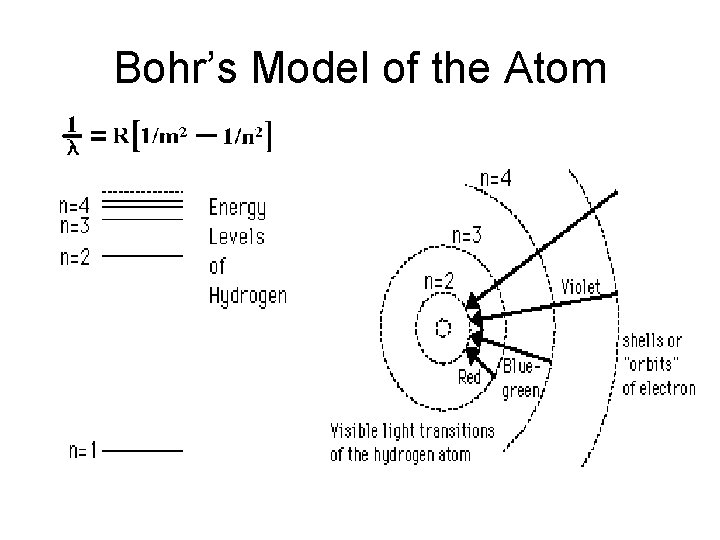
Bohr’s Model of the Atom

ARE WE GOOD SO FAR?

Quantum Model of the Atom • Bohr's model was only good for certain atoms! • A new model was developed– THE QUANTUM MODEL!
The Quantum Model • In 1924 de Broglie showed that electrons had wave-like properties • In 1926, Shrodinger applied “wave” mathematics to the Bohr model of the atom in which the electrons were pictured as particles (matter). • The model which he produced, along with Heisenberg, is essentially a mathematical description of the atom which is difficult for us to visualize in concrete terms.
The Quantum Model • • The quantum model does NOT pinpoint the location of the electron about the nucleus nor does it restrict it to fixed orbits as does the Bohr model. The quantum model gives only PROBABILITY information on the location of the electron relative to the nucleus.

Heisenberg Uncertainty Principle • Heisenberg showed that it is impossible to know both the position and the momentum of electron! • Instead, we can define a "volume" of space (an electron cloud), where there is a high probabilty of finding an electron – these "e- clouds" we call ORBITALS!

ARE YOU WITH ME?
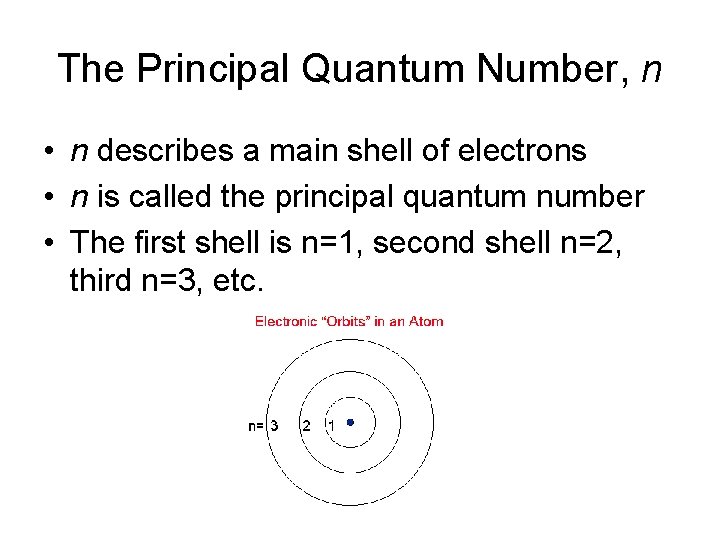
The Principal Quantum Number, n • n describes a main shell of electrons • n is called the principal quantum number • The first shell is n=1, second shell n=2, third n=3, etc.
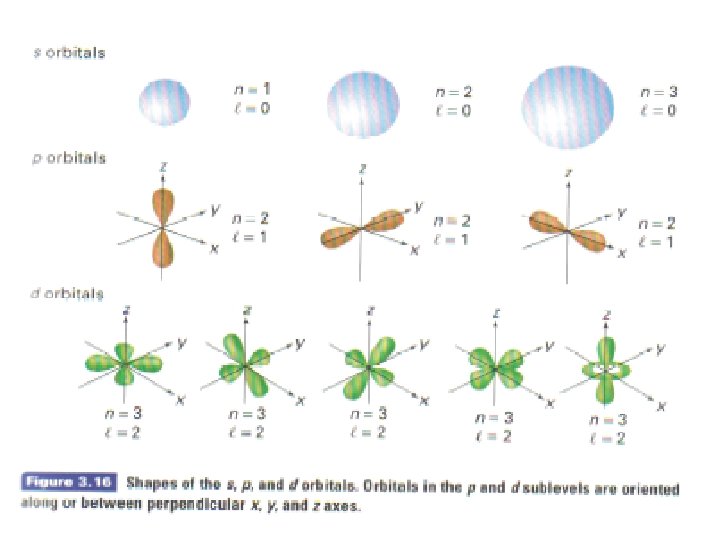
The Secondary Quantum Number, l • l is used to describe additional electron energy sublevels (or subshells). • This means that Bohr’s main energy “step” is actually a group of little “steps” • l relates primarily to the shape of the electron orbital • For n= 1, l = 0 (one subshell); n=2, l = 0, 1 (two subshells) ; n=3, l =0, 1, 2 (three subshells) (l ranges from 0 to n-1)

The Magnetic Quantum Number, ml • ml relates primarily to the direction of the electron orbit • Orbits could exist at various angles • ml can vary from –l to +l • Example: if l=1, them ml can be -1, 0, +1 meaning that there are 3 orbits that have the same energy and shape, but differ in their orientation in space

The Spin Quantum Number, ms • Many substances, elements and compounds are paramagnetic (individual atoms are magnetic) • Evidence suggests that electrons spin on its axis, which acts like a tiny magnet • There are only 2 spins (clockwise or ccw) • The ms can only be +1/2 or -1/2 for any e • An opposite pair of e- spins is a stable arrangement an produces no magnetism
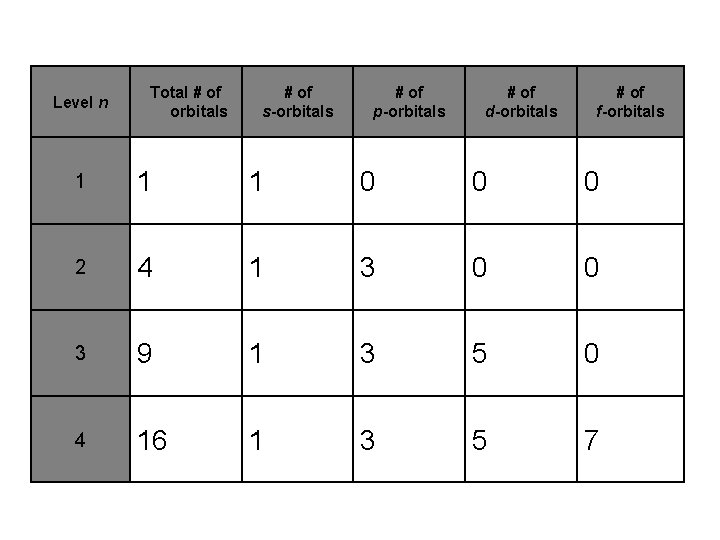
Level n Total # of orbitals # of s-orbitals # of p-orbitals # of d-orbitals # of f-orbitals 1 1 1 0 0 0 2 4 1 3 0 0 3 9 1 3 5 0 4 16 1 3 5 7

Let’s stop here and work • Page 184 # 3, 4, 5
- Slides: 18