Quantum Mechanics and Atomic Theory Dmitri Ivanovich Mendeleev













- Slides: 66

Quantum Mechanics and Atomic Theory

Dmitri Ivanovich Mendeleev Source: Corbis Copyright © Houghton Mifflin Company. All rights reserved. 12 a– 2

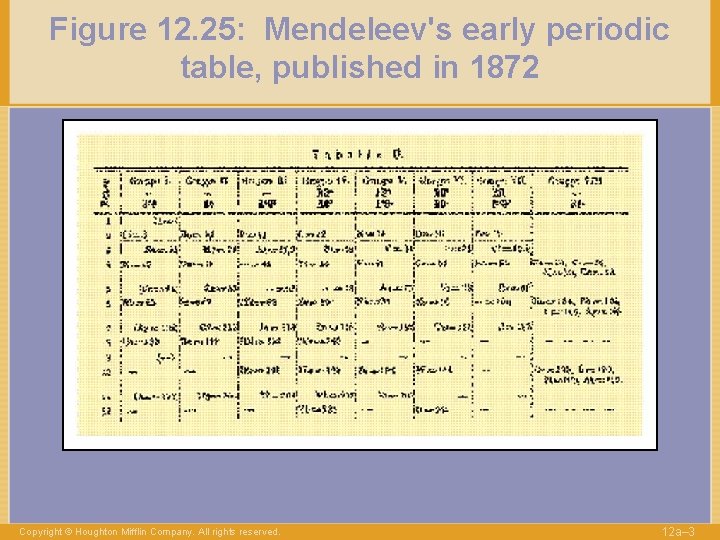
Figure 12. 25: Mendeleev's early periodic table, published in 1872 Copyright © Houghton Mifflin Company. All rights reserved. 12 a– 3

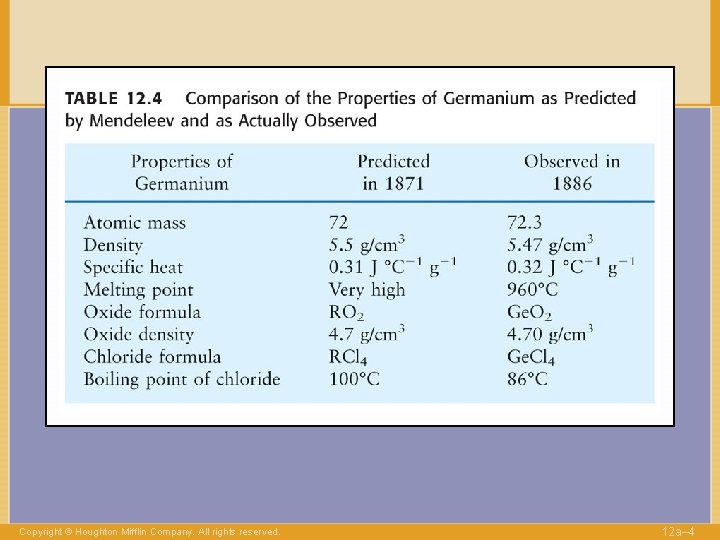
Copyright © Houghton Mifflin Company. All rights reserved. 12 a– 4

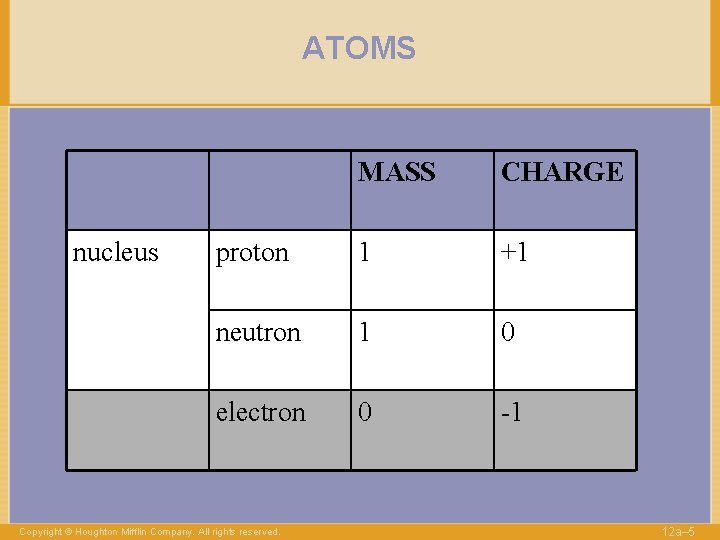
ATOMS nucleus MASS CHARGE proton 1 +1 neutron 1 0 electron 0 -1 Copyright © Houghton Mifflin Company. All rights reserved. 12 a– 5

CHEMISTRY Copyright © Houghton Mifflin Company. All rights reserved. 12 a– 6

CHEMISTRY Most chemistry is in the electrons Copyright © Houghton Mifflin Company. All rights reserved. 12 a– 7

CHEMISTRY Most chemistry is in the electrons (the outer shell electrons) Copyright © Houghton Mifflin Company. All rights reserved. 12 a– 8

CHEMISTRY Most chemistry is in the electrons (the valence electrons) Copyright © Houghton Mifflin Company. All rights reserved. 12 a– 9

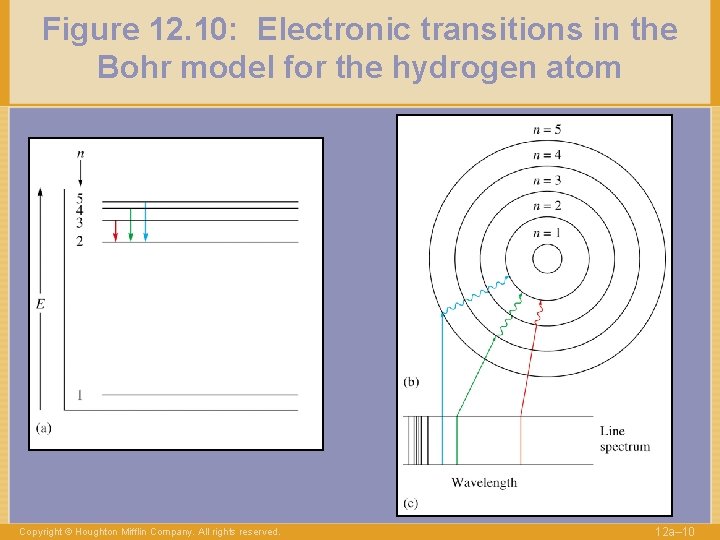
Figure 12. 10: Electronic transitions in the Bohr model for the hydrogen atom Copyright © Houghton Mifflin Company. All rights reserved. 12 a– 10

Copyright © Houghton Mifflin Company. All rights reserved. 12 a– 11

Massive star in Pistol Nebula Source: NASA Copyright © Houghton Mifflin Company. All rights reserved. 12 a– 12

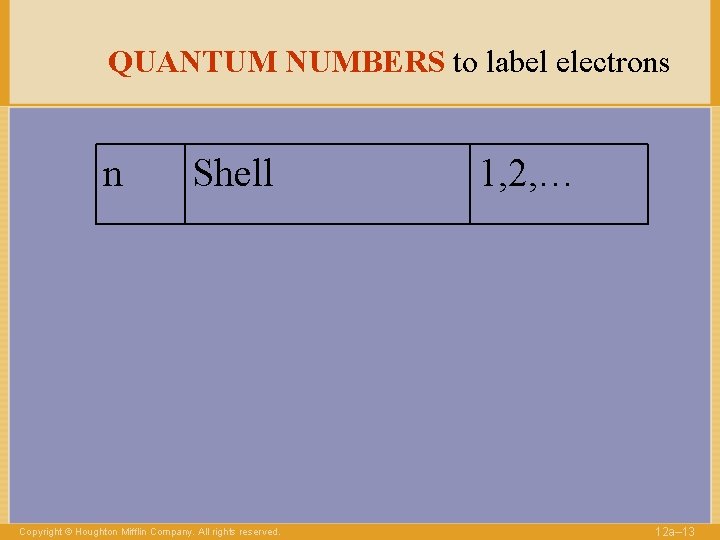
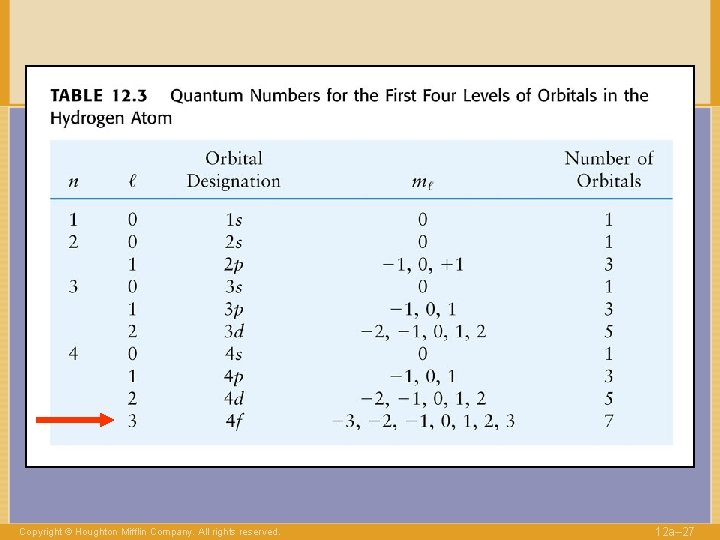
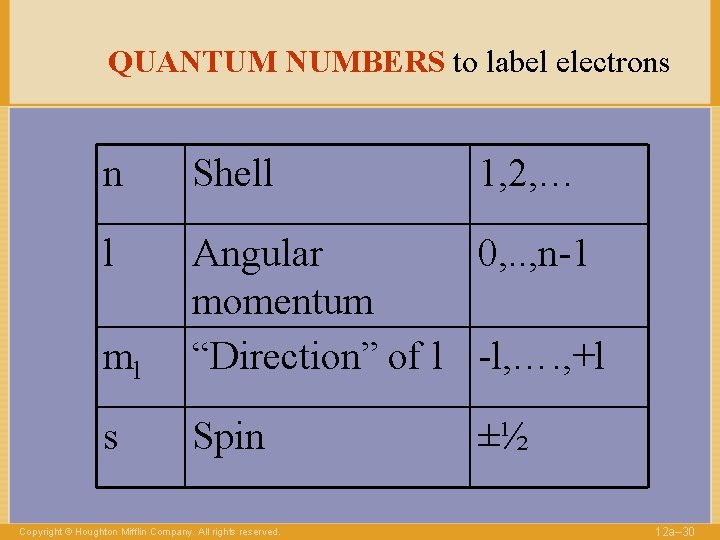
QUANTUM NUMBERS to label electrons n Shell Copyright © Houghton Mifflin Company. All rights reserved. 1, 2, … 12 a– 13

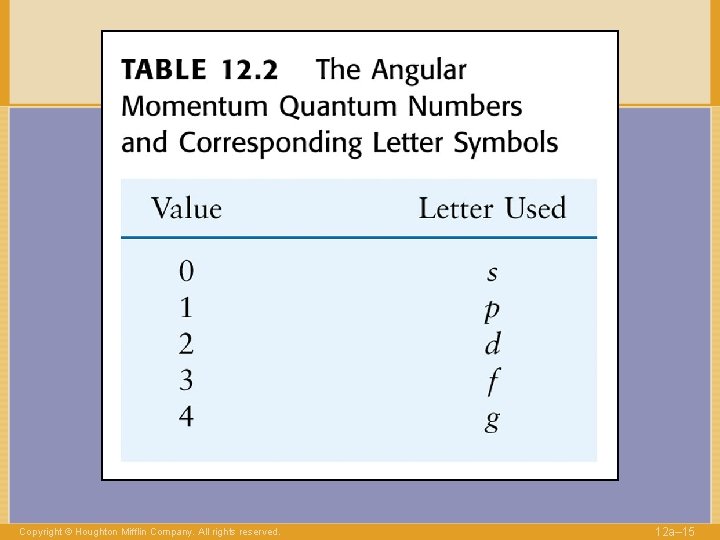
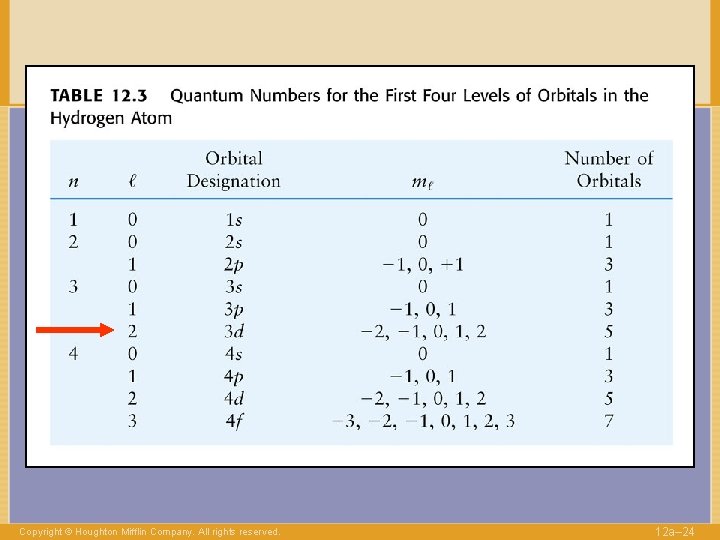
QUANTUM NUMBERS to label electrons n Shell 1, 2, … l Angular momentum 0, . . , n-1 Copyright © Houghton Mifflin Company. All rights reserved. 12 a– 14

Copyright © Houghton Mifflin Company. All rights reserved. 12 a– 15

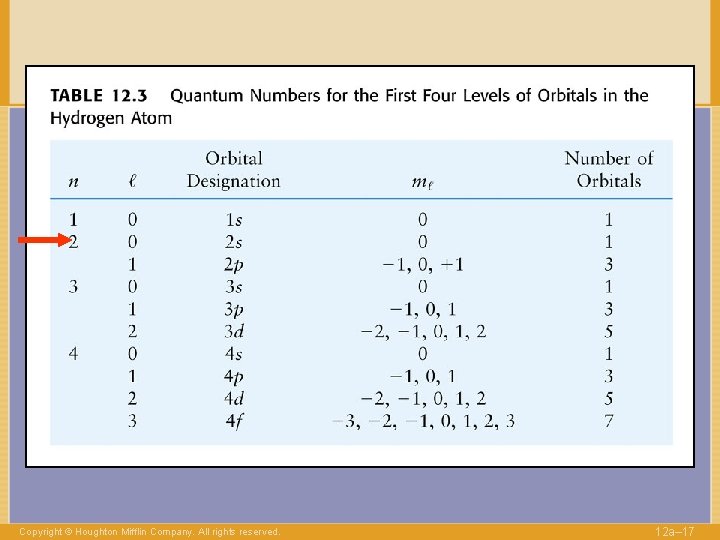
QUANTUM NUMBERS to label electrons n Shell l Angular 0, . . , n-1 momentum “Direction” of l -l, …. , +l ml Copyright © Houghton Mifflin Company. All rights reserved. 1, 2, … 12 a– 16

Copyright © Houghton Mifflin Company. All rights reserved. 12 a– 17

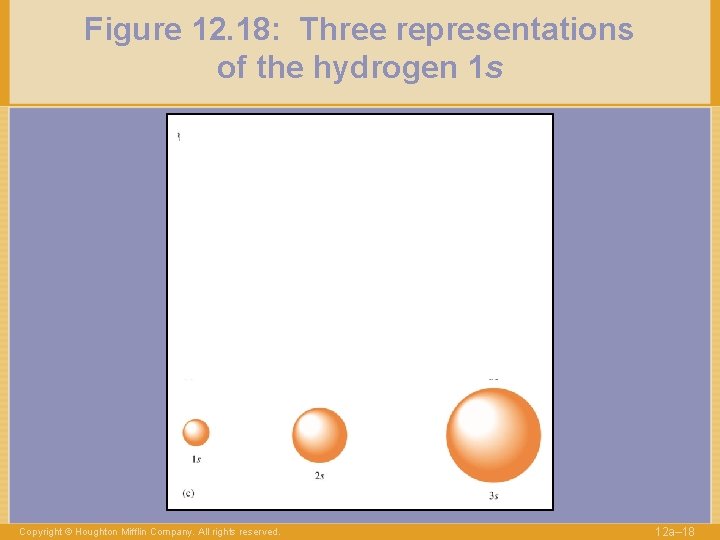
Figure 12. 18: Three representations of the hydrogen 1 s Copyright © Houghton Mifflin Company. All rights reserved. 12 a– 18

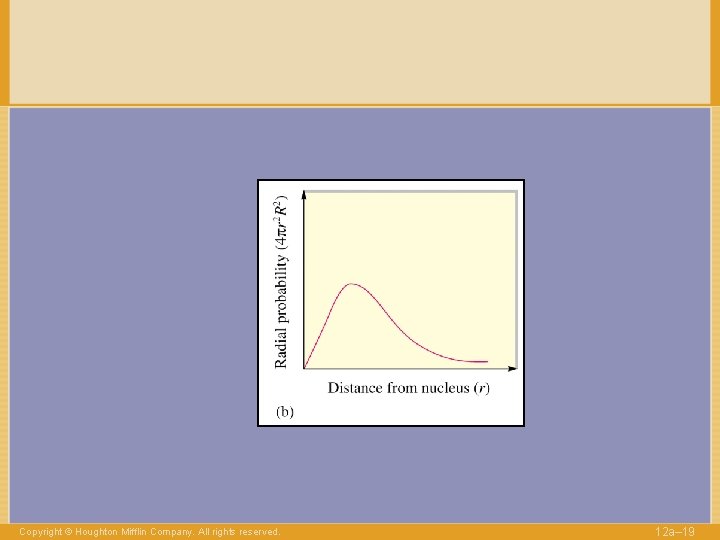
Copyright © Houghton Mifflin Company. All rights reserved. 12 a– 19

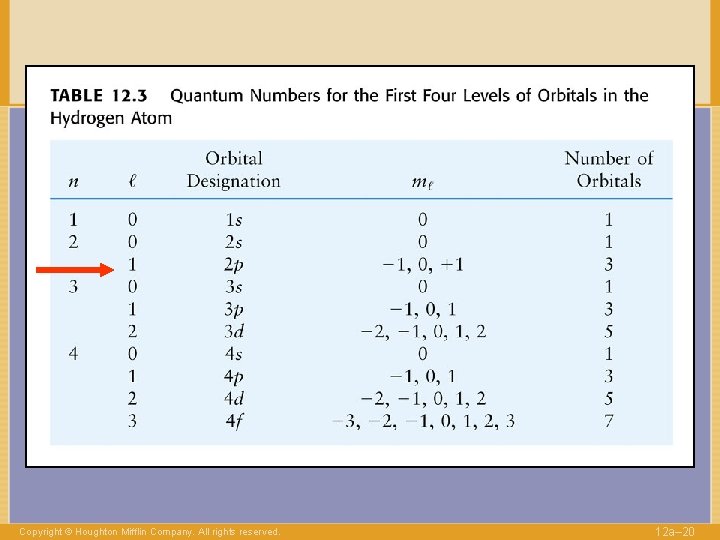
Copyright © Houghton Mifflin Company. All rights reserved. 12 a– 20

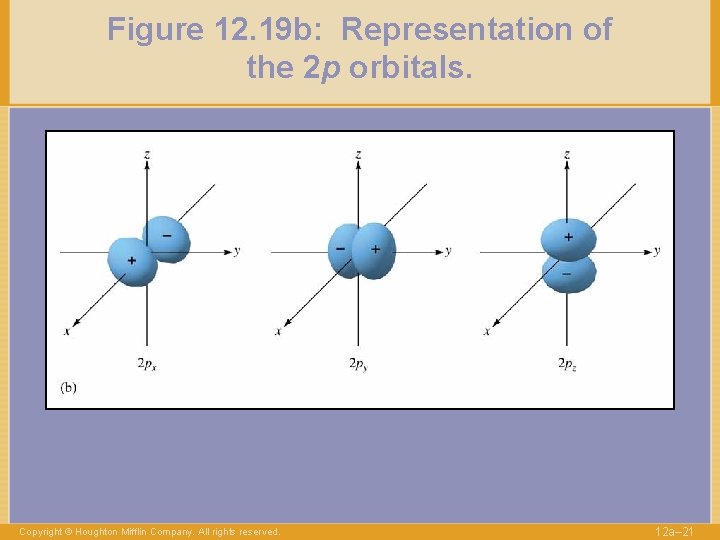
Figure 12. 19 b: Representation of the 2 p orbitals. Copyright © Houghton Mifflin Company. All rights reserved. 12 a– 21

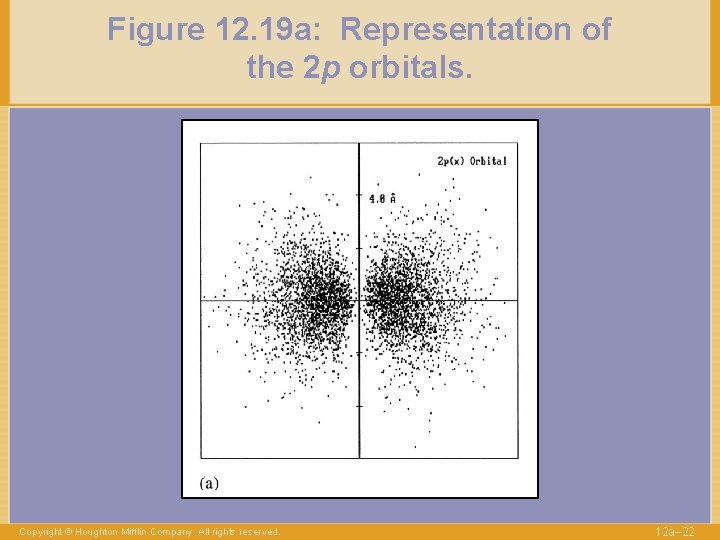
Figure 12. 19 a: Representation of the 2 p orbitals. Copyright © Houghton Mifflin Company. All rights reserved. 12 a– 22

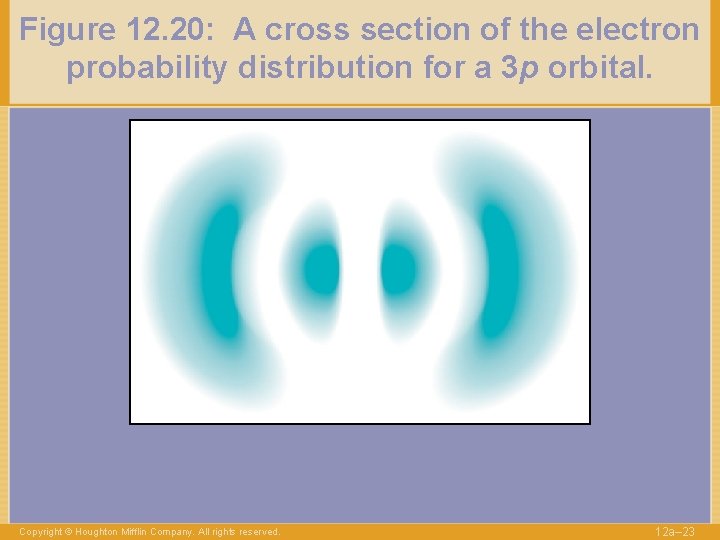
Figure 12. 20: A cross section of the electron probability distribution for a 3 p orbital. Copyright © Houghton Mifflin Company. All rights reserved. 12 a– 23

Copyright © Houghton Mifflin Company. All rights reserved. 12 a– 24

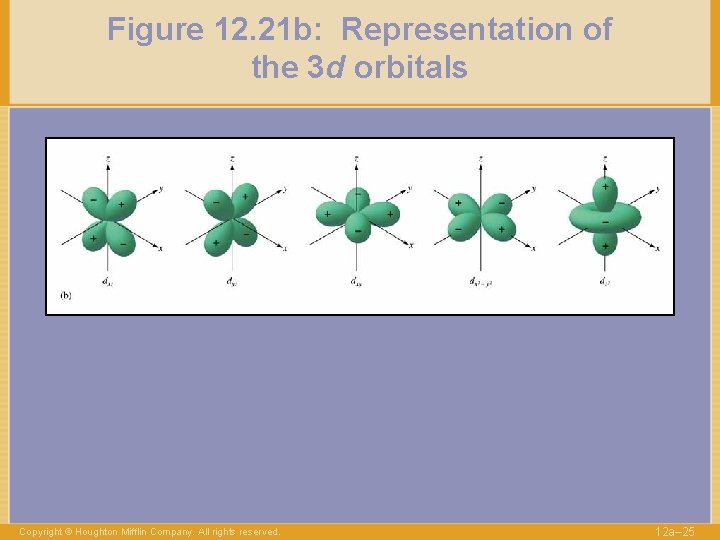
Figure 12. 21 b: Representation of the 3 d orbitals Copyright © Houghton Mifflin Company. All rights reserved. 12 a– 25

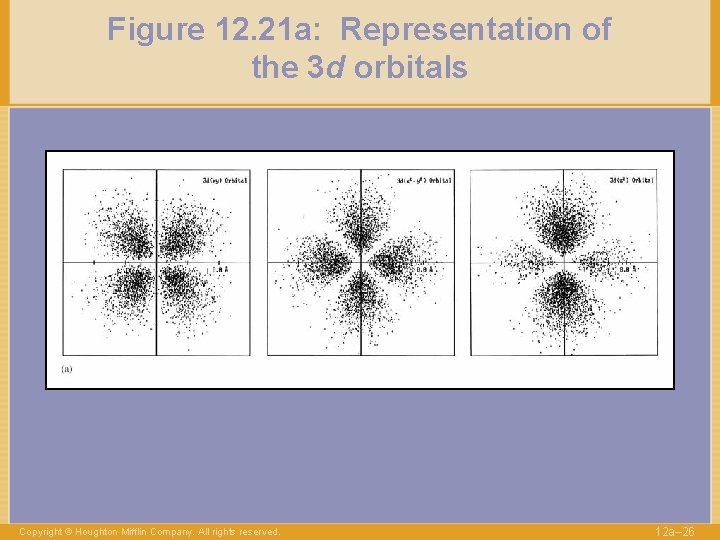
Figure 12. 21 a: Representation of the 3 d orbitals Copyright © Houghton Mifflin Company. All rights reserved. 12 a– 26

Copyright © Houghton Mifflin Company. All rights reserved. 12 a– 27

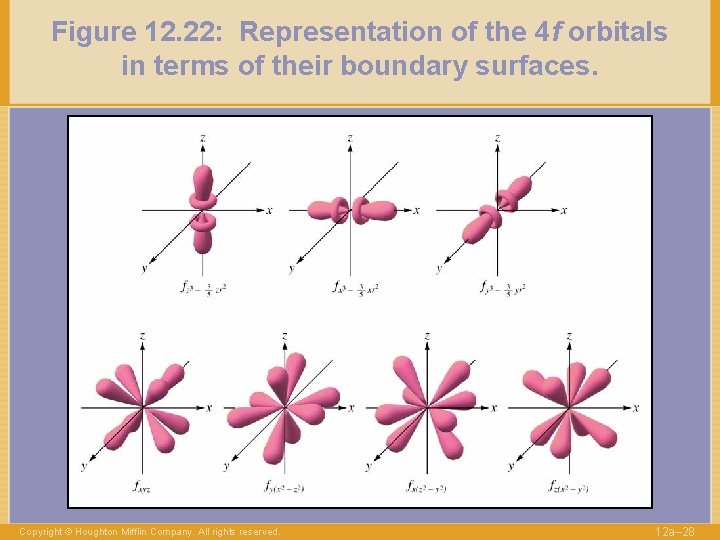
Figure 12. 22: Representation of the 4 f orbitals in terms of their boundary surfaces. Copyright © Houghton Mifflin Company. All rights reserved. 12 a– 28

QUANTUM NUMBERS to label electrons n Shell l Angular 0, . . , n-1 momentum “Direction” of l -l, …. , +l ml Copyright © Houghton Mifflin Company. All rights reserved. 1, 2, … 12 a– 29

QUANTUM NUMBERS to label electrons n Shell l ml Angular 0, . . , n-1 momentum “Direction” of l -l, …. , +l s Spin Copyright © Houghton Mifflin Company. All rights reserved. 1, 2, … ±½ 12 a– 30

Wolfgang Pauli Copyright © Houghton Mifflin Company. All rights reserved. 12 a– 31

Wolfgang Pauli Copyright © Houghton Mifflin Company. All rights reserved. 12 a– 32

PAULI PRINCIPLE • NO TWO ELECTRONS SHALL HAVE THE SAME SET OF QUANTUM NUMBERS Copyright © Houghton Mifflin Company. All rights reserved. 12 a– 33

PAULI PRINCIPLE • NO TWO ELECTRONS SHALL HAVE THE SAME SET OF QUANTUM NUMBERS • Consequence: AUFBAU Copyright © Houghton Mifflin Company. All rights reserved. 12 a– 34

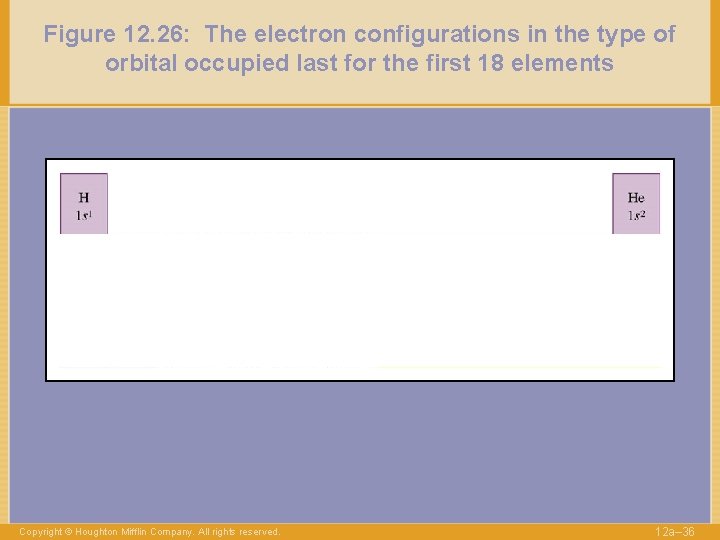
Figure 12. 26: The electron configurations in the type of orbital occupied last for the first 18 elements Copyright © Houghton Mifflin Company. All rights reserved. 12 a– 35

Figure 12. 26: The electron configurations in the type of orbital occupied last for the first 18 elements Copyright © Houghton Mifflin Company. All rights reserved. 12 a– 36

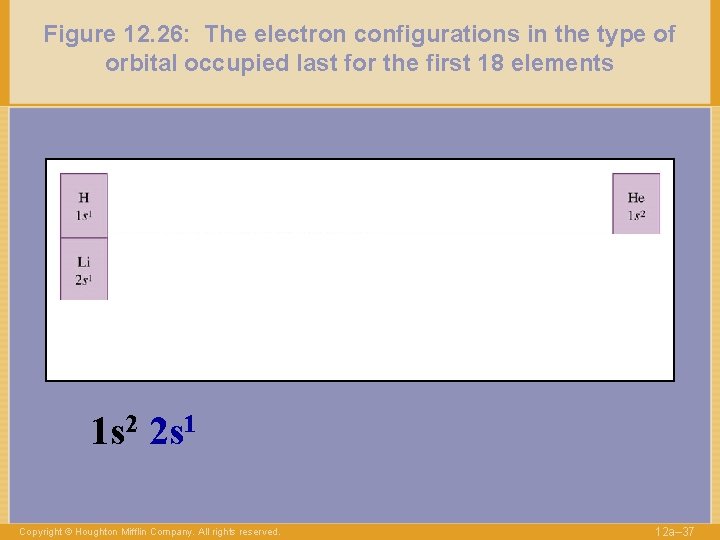
Figure 12. 26: The electron configurations in the type of orbital occupied last for the first 18 elements 1 s 2 2 s 1 Copyright © Houghton Mifflin Company. All rights reserved. 12 a– 37

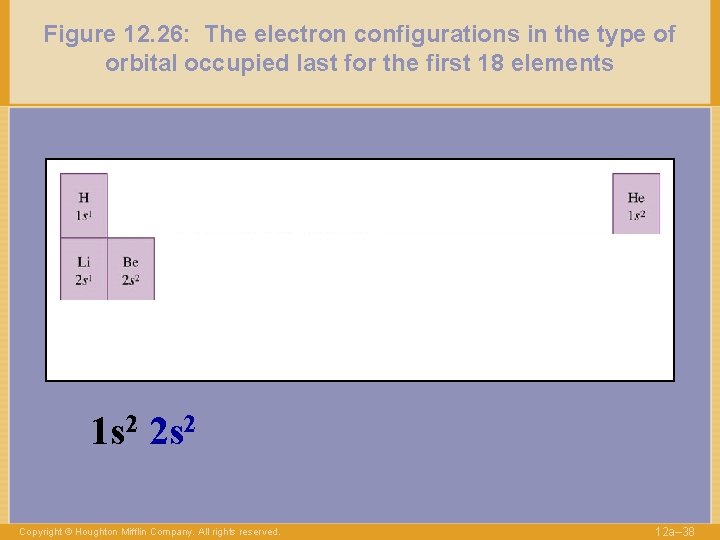
Figure 12. 26: The electron configurations in the type of orbital occupied last for the first 18 elements 1 s 2 2 s 2 Copyright © Houghton Mifflin Company. All rights reserved. 12 a– 38

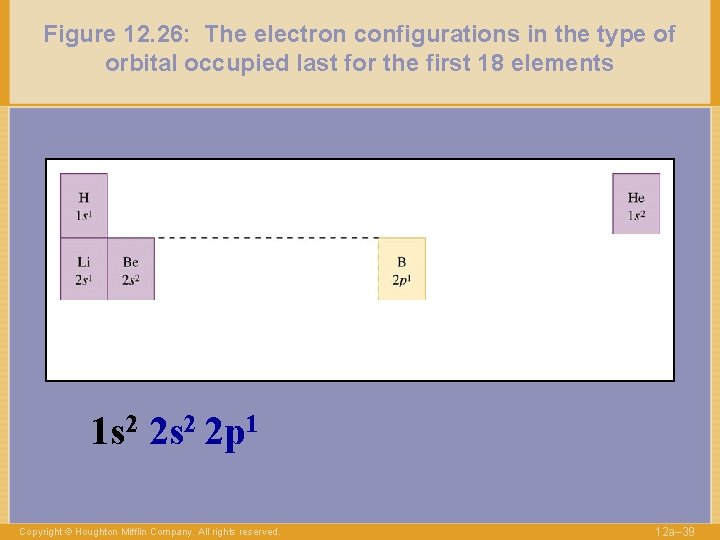
Figure 12. 26: The electron configurations in the type of orbital occupied last for the first 18 elements 1 s 2 2 p 1 Copyright © Houghton Mifflin Company. All rights reserved. 12 a– 39

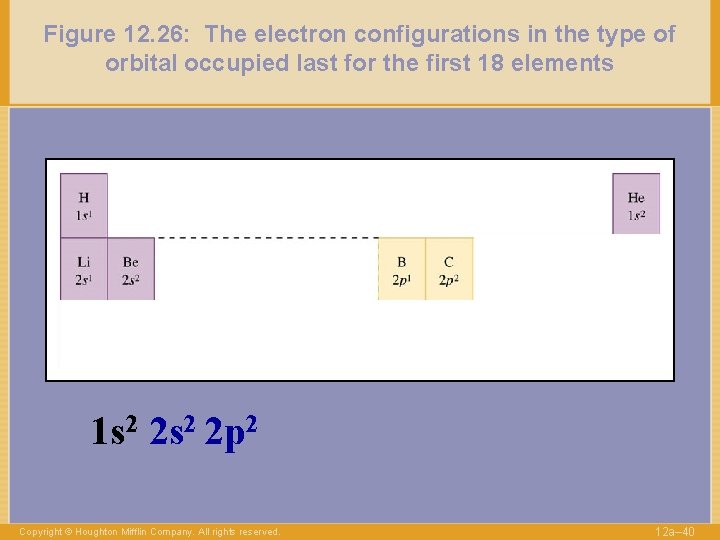
Figure 12. 26: The electron configurations in the type of orbital occupied last for the first 18 elements 1 s 2 2 p 2 Copyright © Houghton Mifflin Company. All rights reserved. 12 a– 40

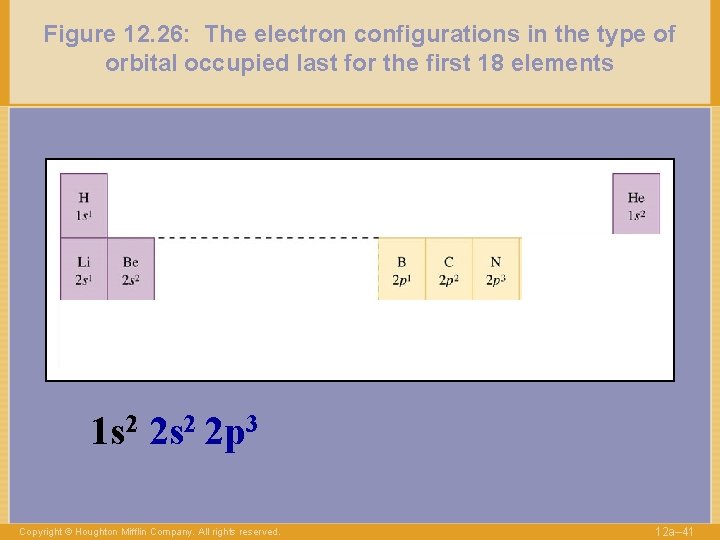
Figure 12. 26: The electron configurations in the type of orbital occupied last for the first 18 elements 1 s 2 2 p 3 Copyright © Houghton Mifflin Company. All rights reserved. 12 a– 41

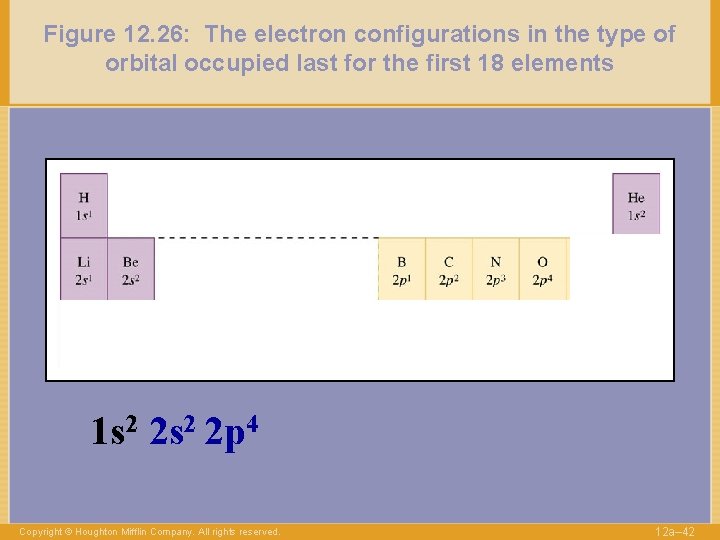
Figure 12. 26: The electron configurations in the type of orbital occupied last for the first 18 elements 1 s 2 2 p 4 Copyright © Houghton Mifflin Company. All rights reserved. 12 a– 42

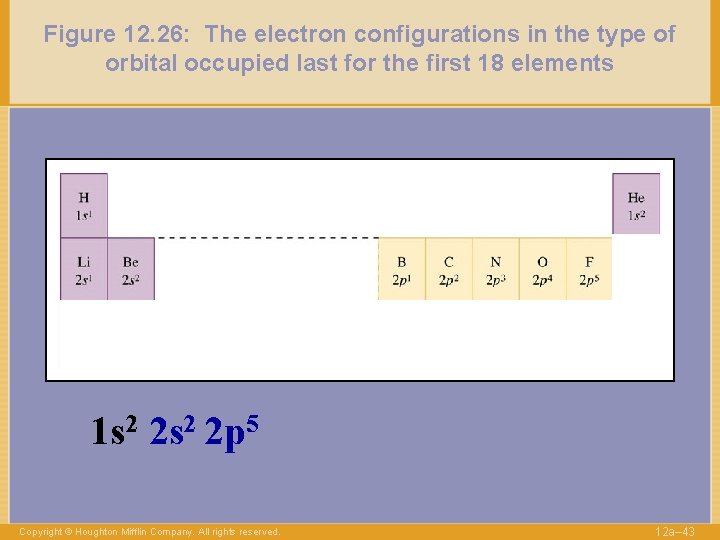
Figure 12. 26: The electron configurations in the type of orbital occupied last for the first 18 elements 1 s 2 2 p 5 Copyright © Houghton Mifflin Company. All rights reserved. 12 a– 43

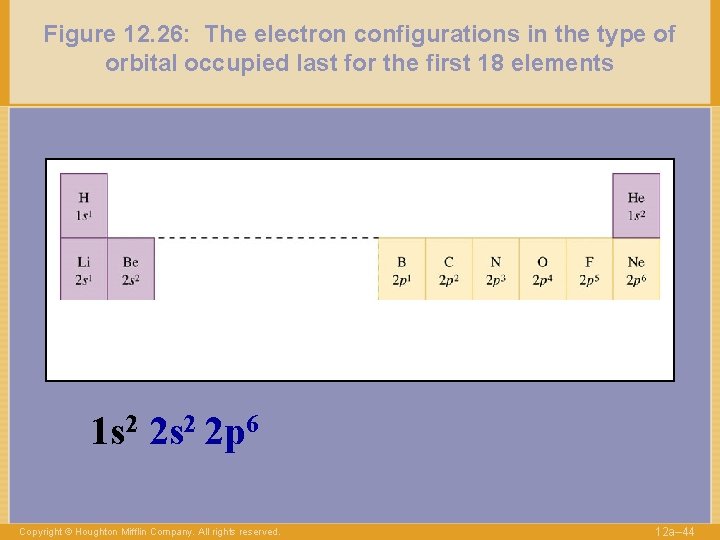
Figure 12. 26: The electron configurations in the type of orbital occupied last for the first 18 elements 1 s 2 2 p 6 Copyright © Houghton Mifflin Company. All rights reserved. 12 a– 44

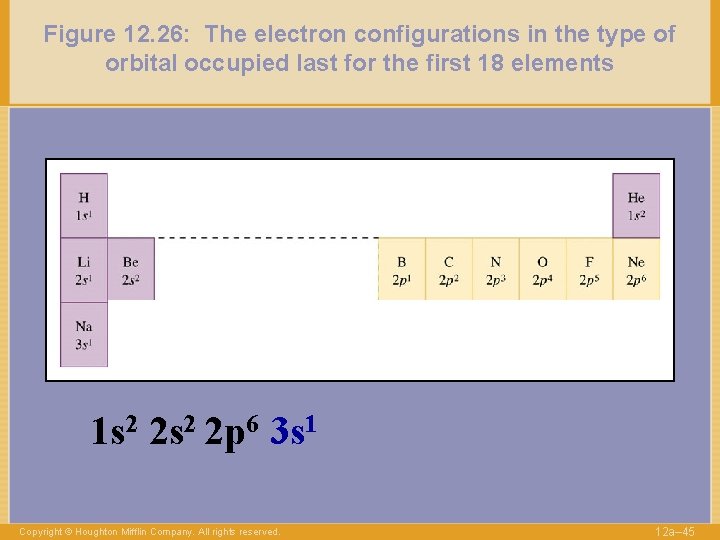
Figure 12. 26: The electron configurations in the type of orbital occupied last for the first 18 elements 1 s 2 2 p 6 3 s 1 Copyright © Houghton Mifflin Company. All rights reserved. 12 a– 45

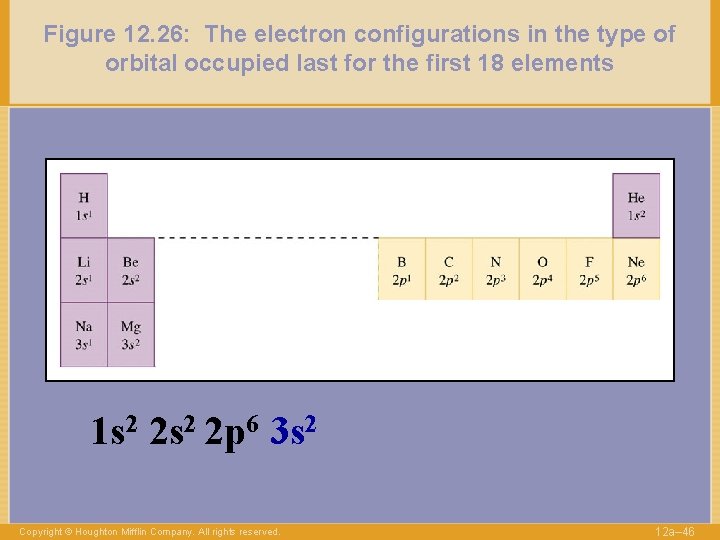
Figure 12. 26: The electron configurations in the type of orbital occupied last for the first 18 elements 1 s 2 2 p 6 3 s 2 Copyright © Houghton Mifflin Company. All rights reserved. 12 a– 46

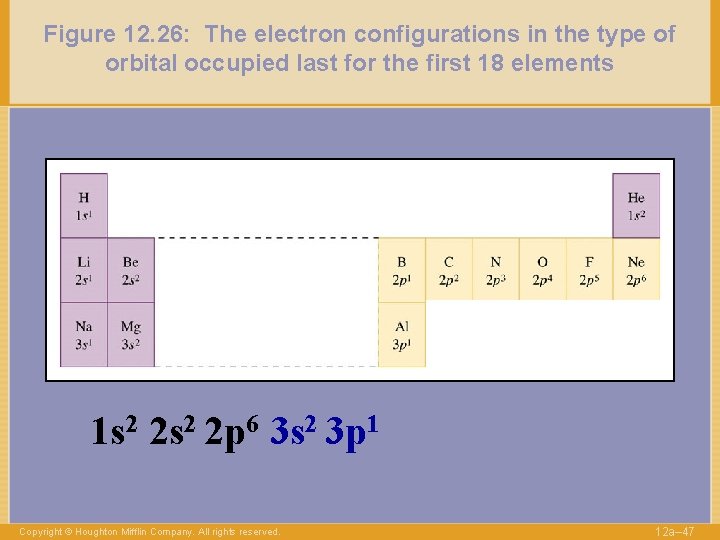
Figure 12. 26: The electron configurations in the type of orbital occupied last for the first 18 elements 1 s 2 2 p 6 3 s 2 3 p 1 Copyright © Houghton Mifflin Company. All rights reserved. 12 a– 47

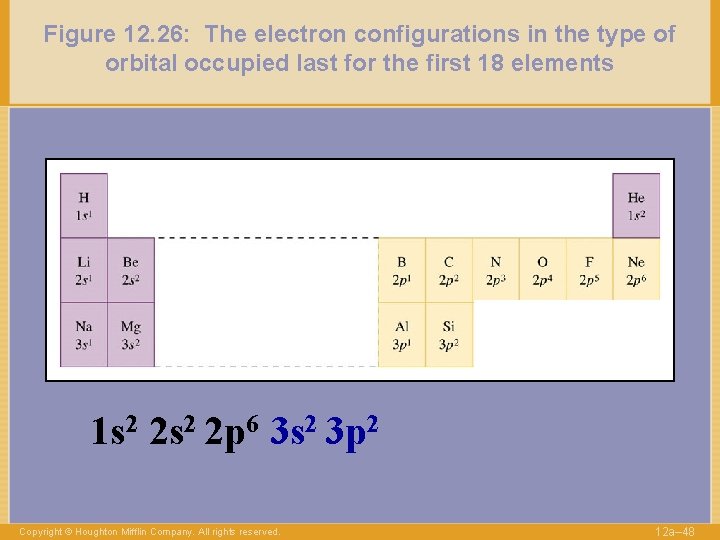
Figure 12. 26: The electron configurations in the type of orbital occupied last for the first 18 elements 1 s 2 2 p 6 3 s 2 3 p 2 Copyright © Houghton Mifflin Company. All rights reserved. 12 a– 48

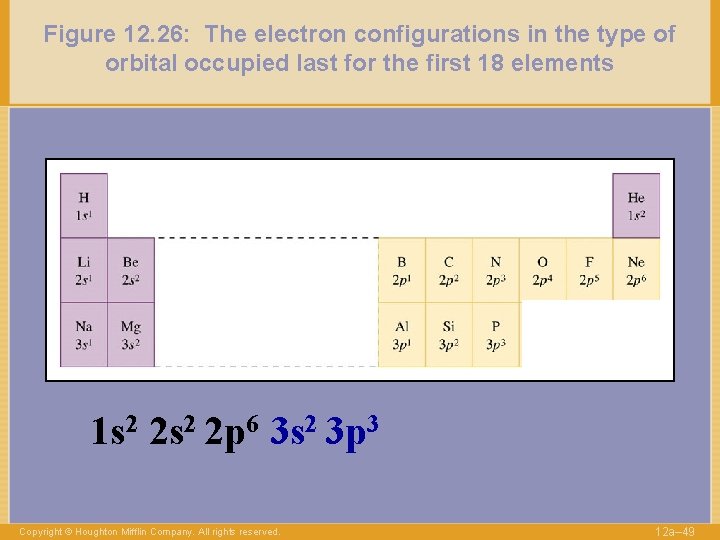
Figure 12. 26: The electron configurations in the type of orbital occupied last for the first 18 elements 1 s 2 2 p 6 3 s 2 3 p 3 Copyright © Houghton Mifflin Company. All rights reserved. 12 a– 49

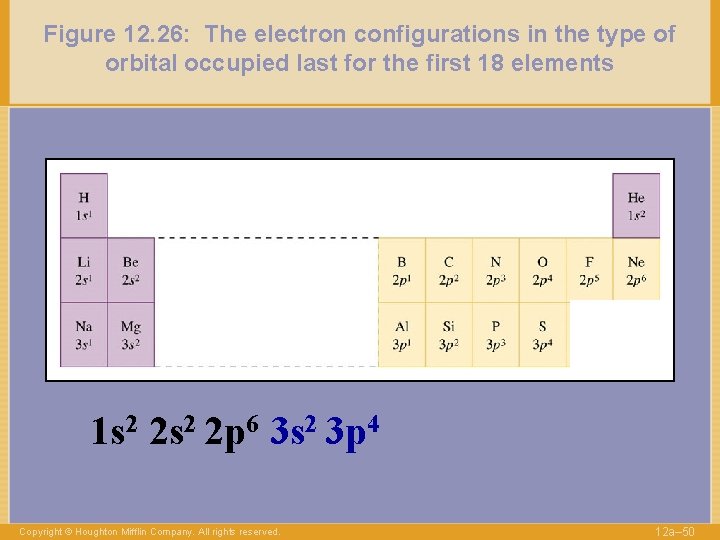
Figure 12. 26: The electron configurations in the type of orbital occupied last for the first 18 elements 1 s 2 2 p 6 3 s 2 3 p 4 Copyright © Houghton Mifflin Company. All rights reserved. 12 a– 50

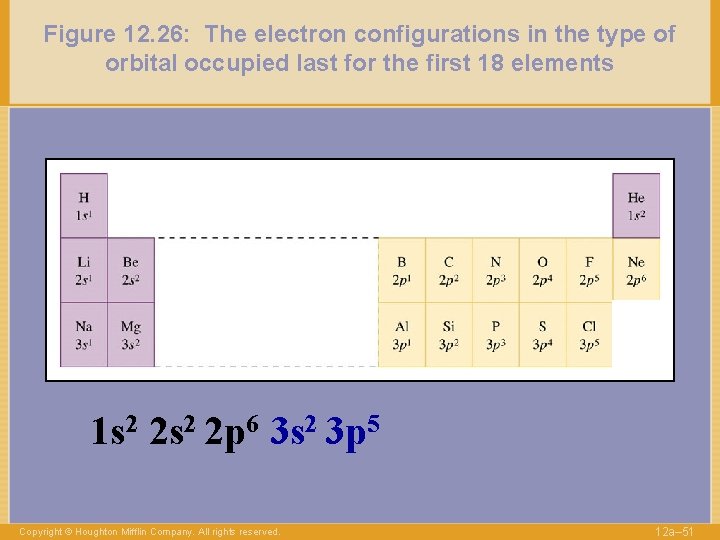
Figure 12. 26: The electron configurations in the type of orbital occupied last for the first 18 elements 1 s 2 2 p 6 3 s 2 3 p 5 Copyright © Houghton Mifflin Company. All rights reserved. 12 a– 51

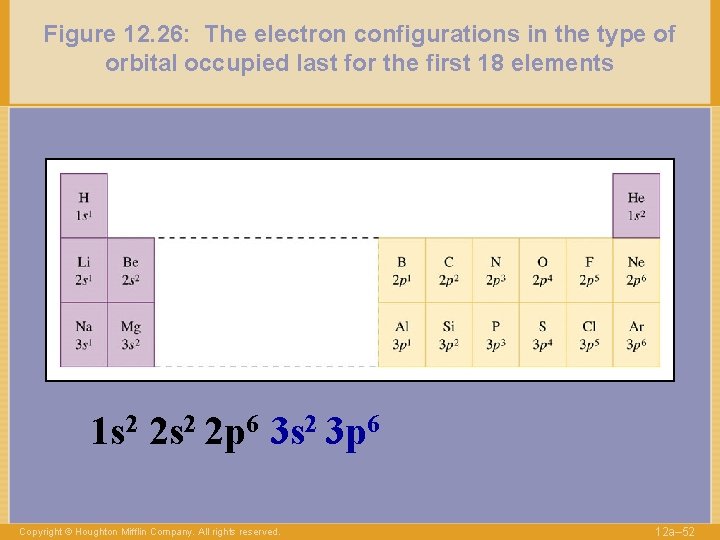
Figure 12. 26: The electron configurations in the type of orbital occupied last for the first 18 elements 1 s 2 2 p 6 3 s 2 3 p 6 Copyright © Houghton Mifflin Company. All rights reserved. 12 a– 52

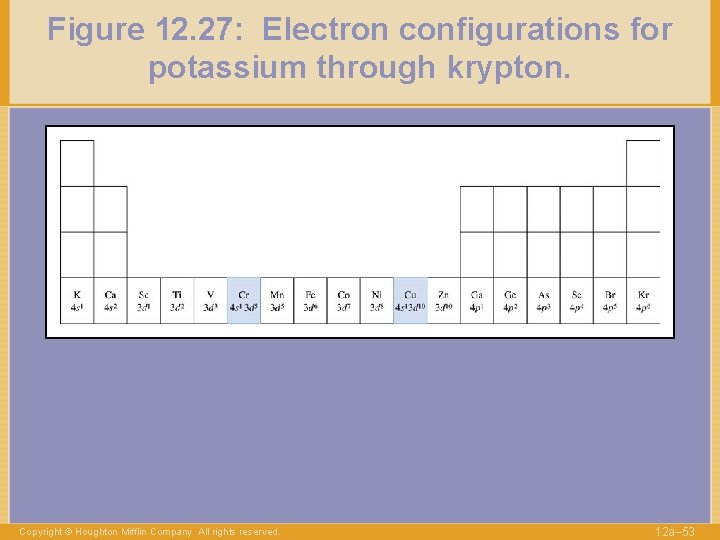
Figure 12. 27: Electron configurations for potassium through krypton. Copyright © Houghton Mifflin Company. All rights reserved. 12 a– 53

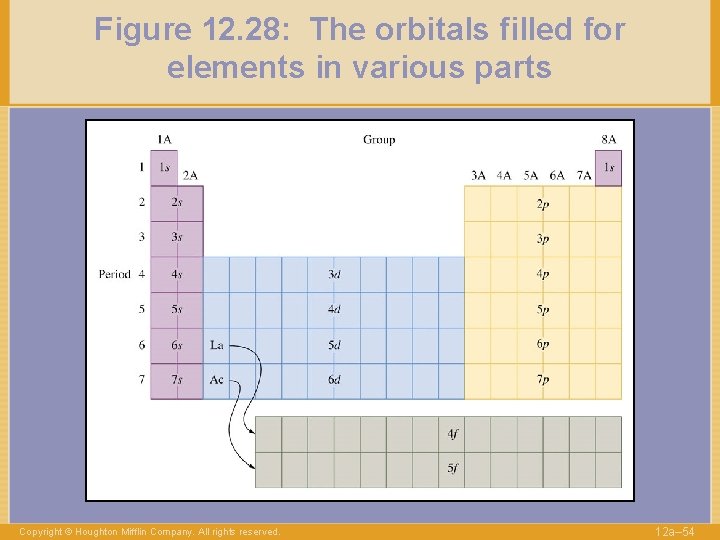
Figure 12. 28: The orbitals filled for elements in various parts Copyright © Houghton Mifflin Company. All rights reserved. 12 a– 54

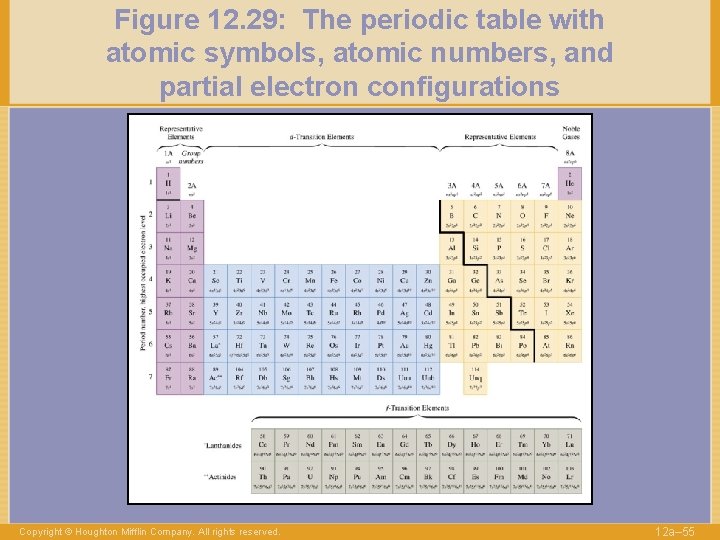
Figure 12. 29: The periodic table with atomic symbols, atomic numbers, and partial electron configurations Copyright © Houghton Mifflin Company. All rights reserved. 12 a– 55

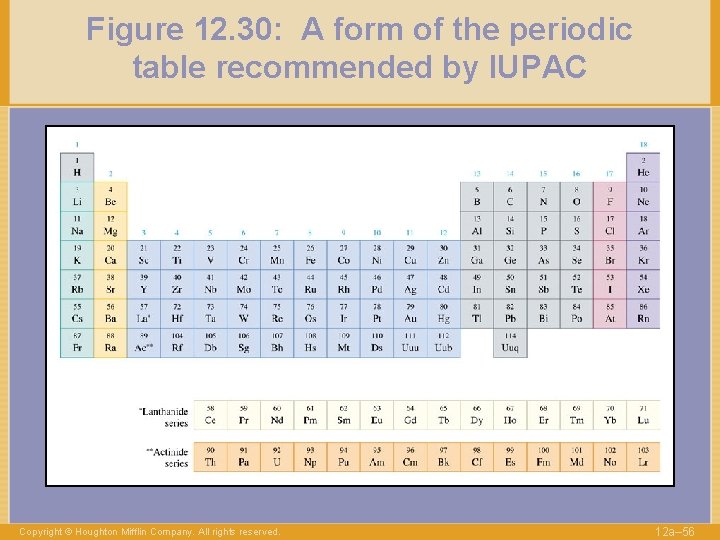
Figure 12. 30: A form of the periodic table recommended by IUPAC Copyright © Houghton Mifflin Company. All rights reserved. 12 a– 56

CHEMISTRY Full shells make the happiest atoms Copyright © Houghton Mifflin Company. All rights reserved. 12 a– 57

CHEMISTRY Full shells make the most stable atoms Copyright © Houghton Mifflin Company. All rights reserved. 12 a– 58

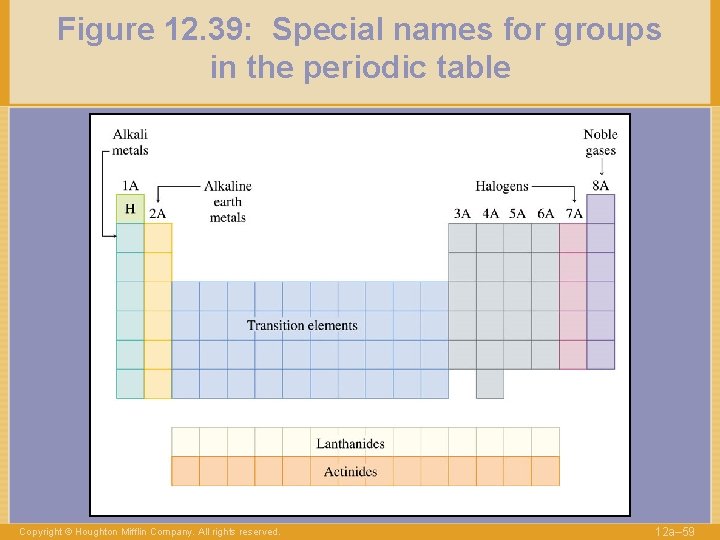
Figure 12. 39: Special names for groups in the periodic table Copyright © Houghton Mifflin Company. All rights reserved. 12 a– 59

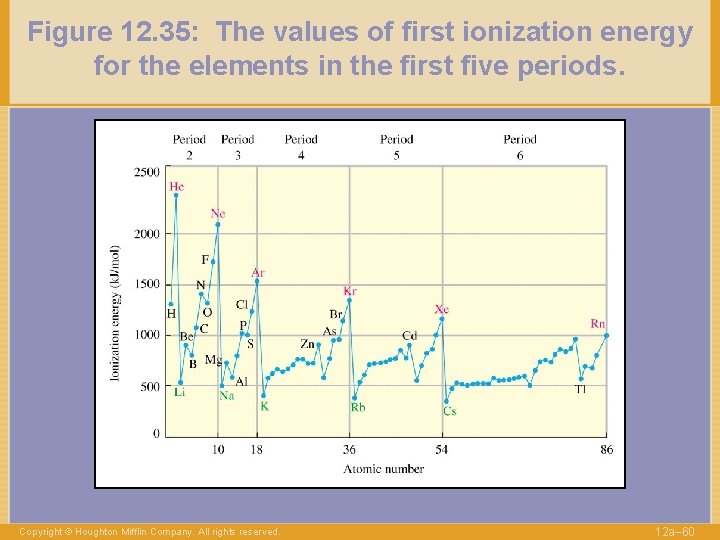
Figure 12. 35: The values of first ionization energy for the elements in the first five periods. Copyright © Houghton Mifflin Company. All rights reserved. 12 a– 60

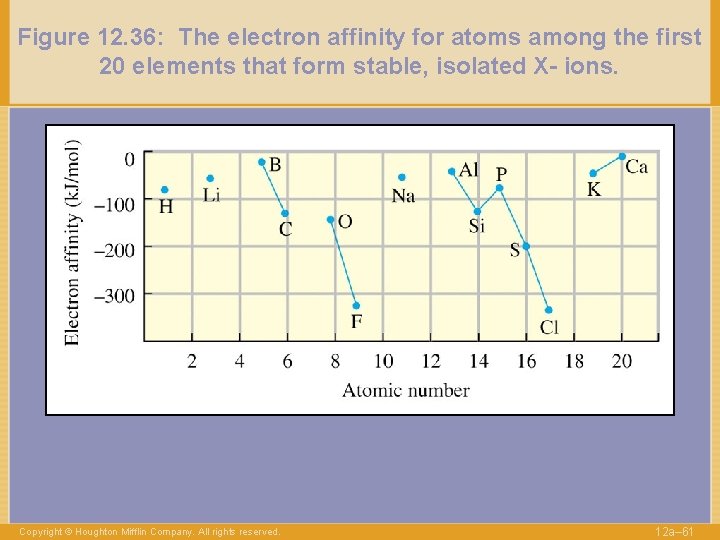
Figure 12. 36: The electron affinity for atoms among the first 20 elements that form stable, isolated X- ions. Copyright © Houghton Mifflin Company. All rights reserved. 12 a– 61

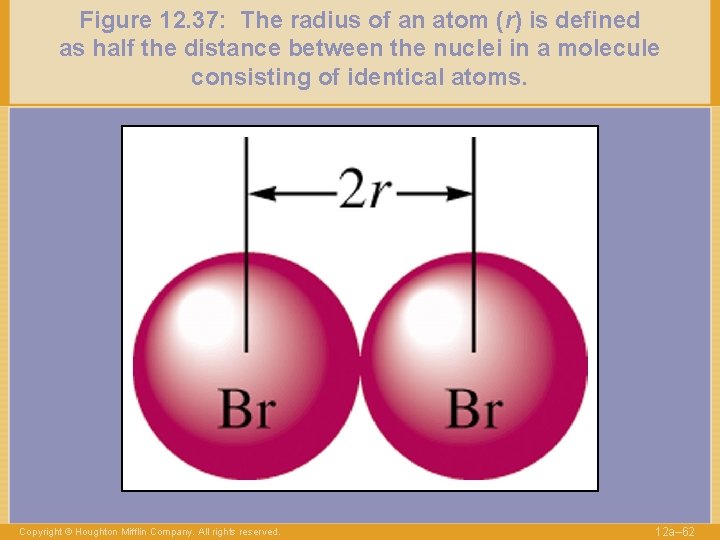
Figure 12. 37: The radius of an atom (r) is defined as half the distance between the nuclei in a molecule consisting of identical atoms. Copyright © Houghton Mifflin Company. All rights reserved. 12 a– 62

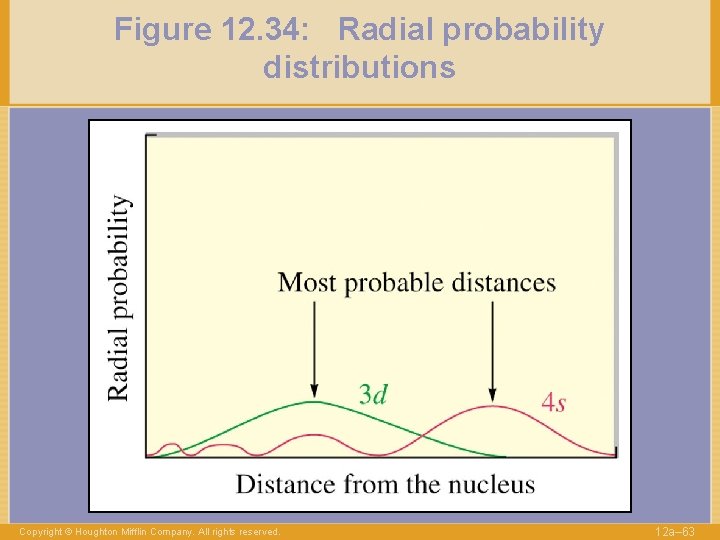
Figure 12. 34: Radial probability distributions Copyright © Houghton Mifflin Company. All rights reserved. 12 a– 63

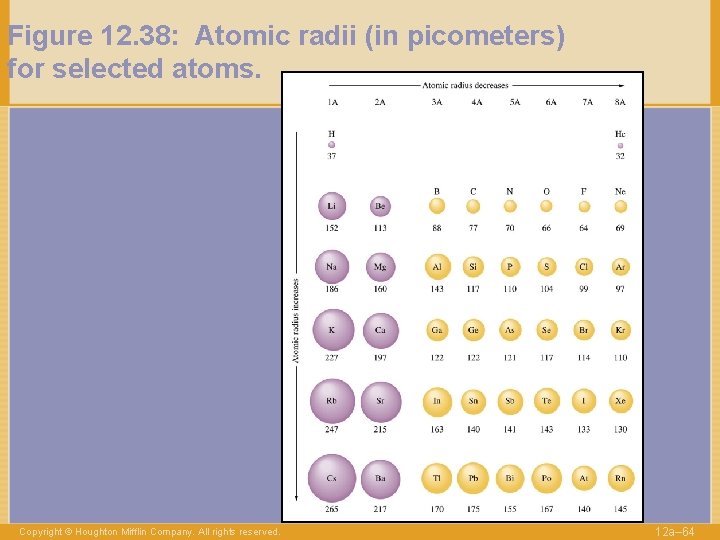
Figure 12. 38: Atomic radii (in picometers) for selected atoms. Copyright © Houghton Mifflin Company. All rights reserved. 12 a– 64

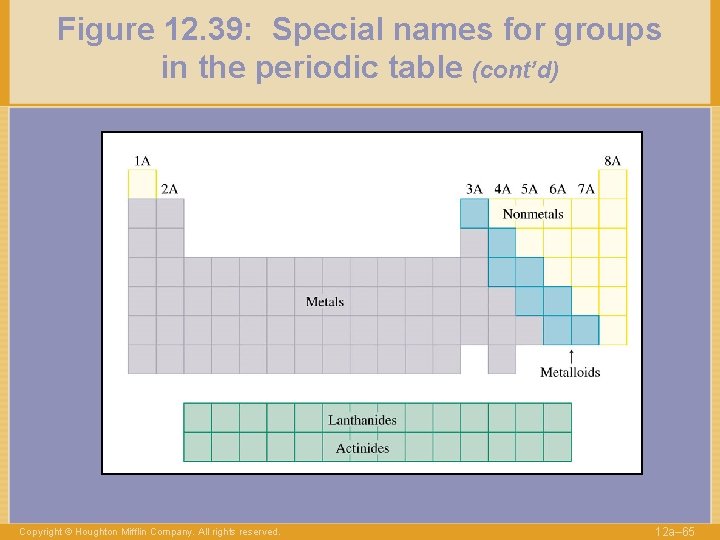
Figure 12. 39: Special names for groups in the periodic table (cont’d) Copyright © Houghton Mifflin Company. All rights reserved. 12 a– 65

Copyright © Houghton Mifflin Company. All rights reserved. 12 a– 66