Quantum Mechanics and Atomic Orbitals Bohr and Einstein
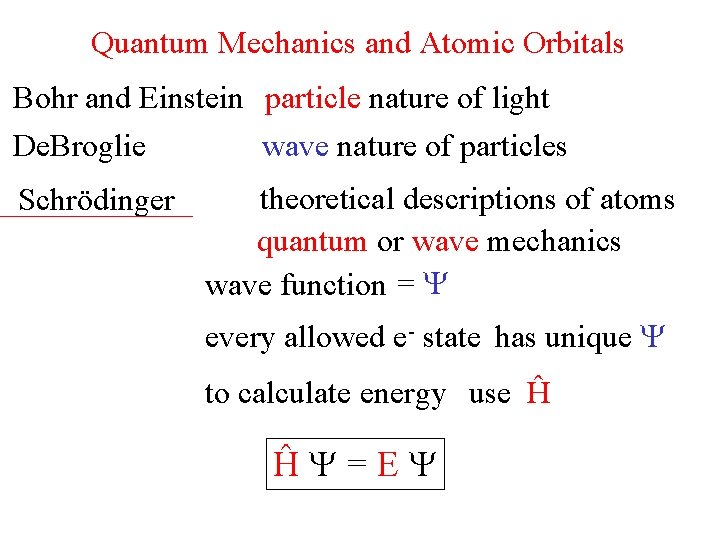
Quantum Mechanics and Atomic Orbitals Bohr and Einstein particle nature of light De. Broglie wave nature of particles Schrödinger theoretical descriptions of atoms quantum or wave mechanics wave function = every allowed e- state has unique to calculate energy use Ĥ Ĥ =E
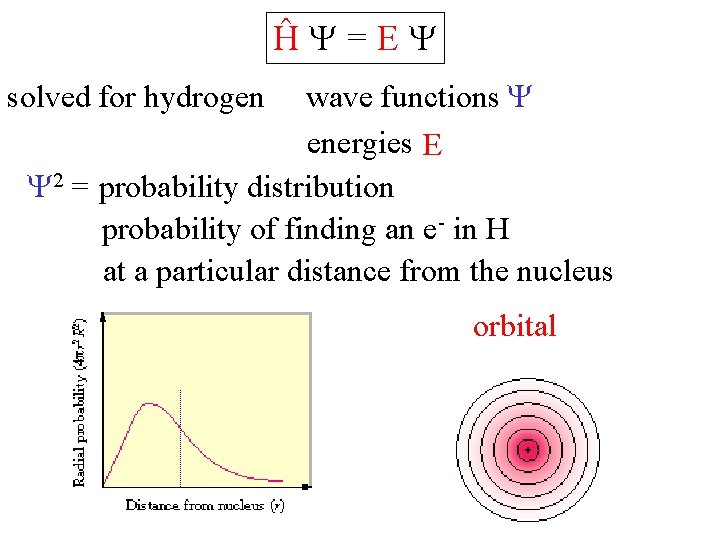
Ĥ =E wave functions energies E 2 = probability distribution probability of finding an e- in H at a particular distance from the nucleus solved for hydrogen orbital
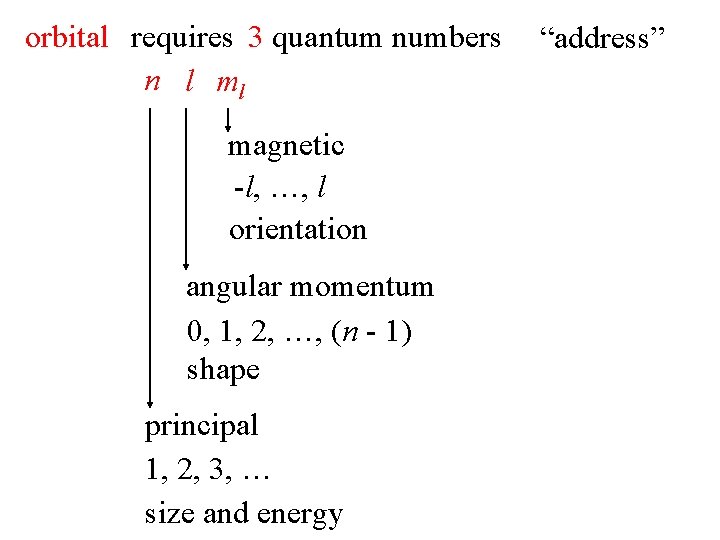
orbital requires 3 quantum numbers n l ml magnetic -l, …, l orientation angular momentum 0, 1, 2, …, (n - 1) shape principal 1, 2, 3, … size and energy “address”
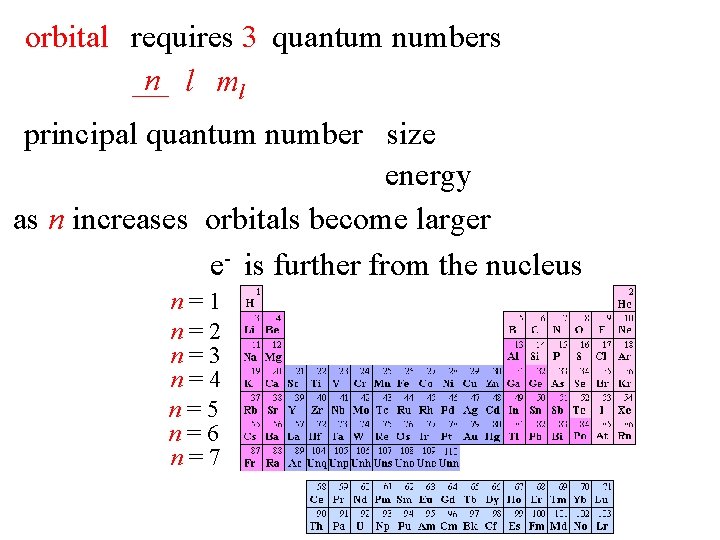
orbital requires 3 quantum numbers n l ml principal quantum number size energy as n increases orbitals become larger e- is further from the nucleus n=1 n=2 n=3 n=4 n=5 n=6 n=7
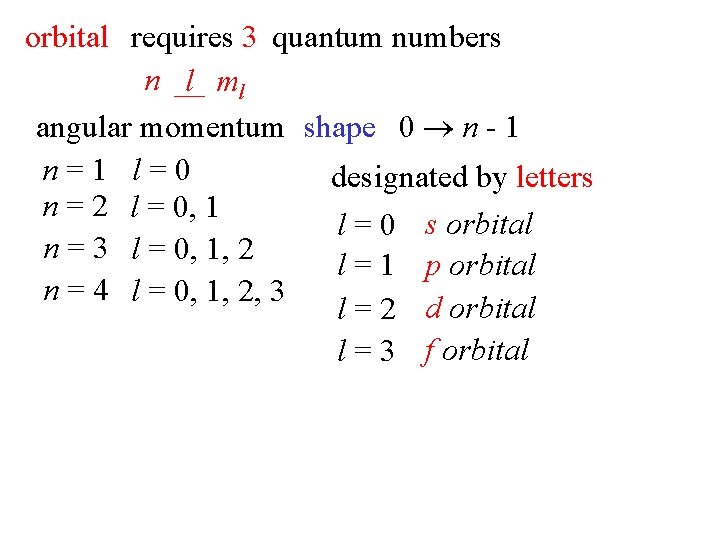
orbital requires 3 quantum numbers n l ml angular momentum shape 0 n - 1 n=1 l=0 designated by letters n = 2 l = 0, 1 l = 0 s orbital n = 3 l = 0, 1, 2 l = 1 p orbital n = 4 l = 0, 1, 2, 3 l = 2 d orbital l = 3 f orbital
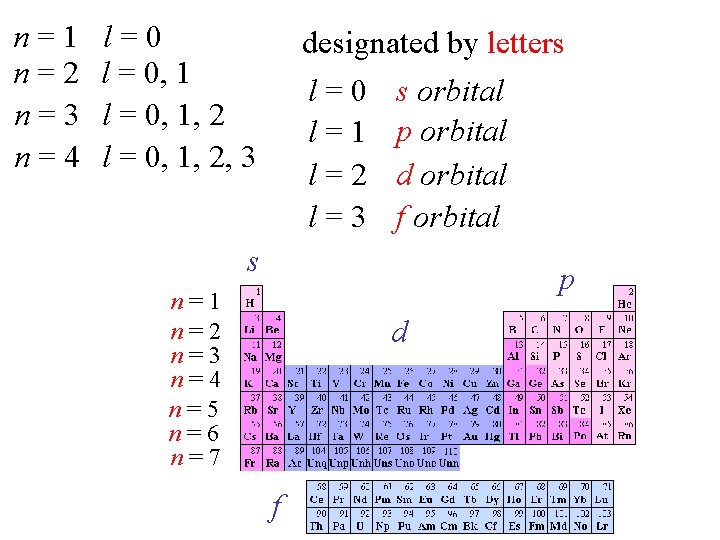
n=1 n=2 n=3 n=4 l=0 l = 0, 1, 2, 3 designated by letters l = 0 s orbital l = 1 p orbital l = 2 d orbital l = 3 f orbital s p n=1 n=2 n=3 n=4 n=5 n=6 n=7 d f

orbital requires 3 quantum numbers n l ml magnetic quantum number -l, …, l s row s n=3 l=0 n=1 l=0 m=0 1 p s l=1 n=2 l=0 m=0 1 p l = 1 m = -1 d m=0 3 l=2 m=1 1 s orbital 3 p orbitals 5 d orbitals m=0 1 m = -1 m=0 3 m=1 m = -2 m = -1 5 m=0 m=1 m=2
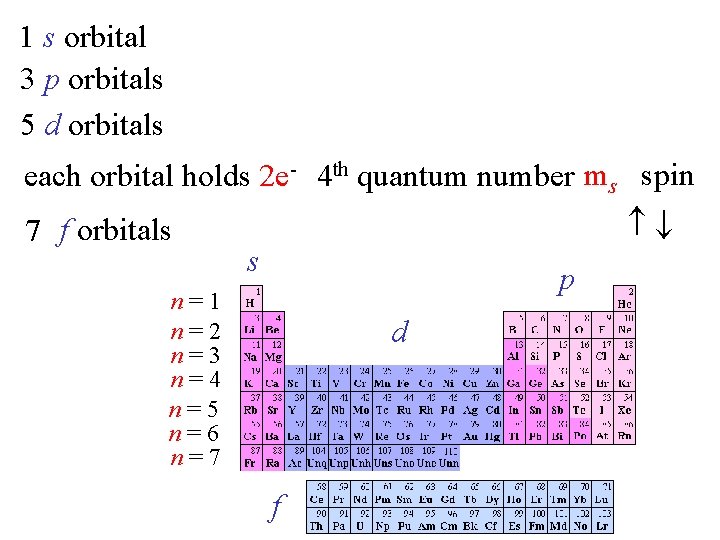
1 s orbital 3 p orbitals 5 d orbitals each orbital holds 2 e- 4 th quantum number ms spin 7 f orbitals s p n=1 n=2 n=3 n=4 n=5 n=6 n=7 d f
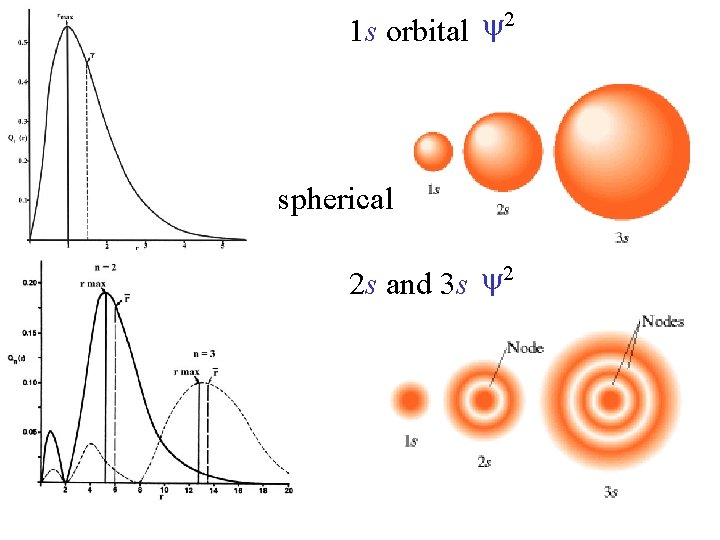
2 1 s orbital spherical 2 2 s and 3 s
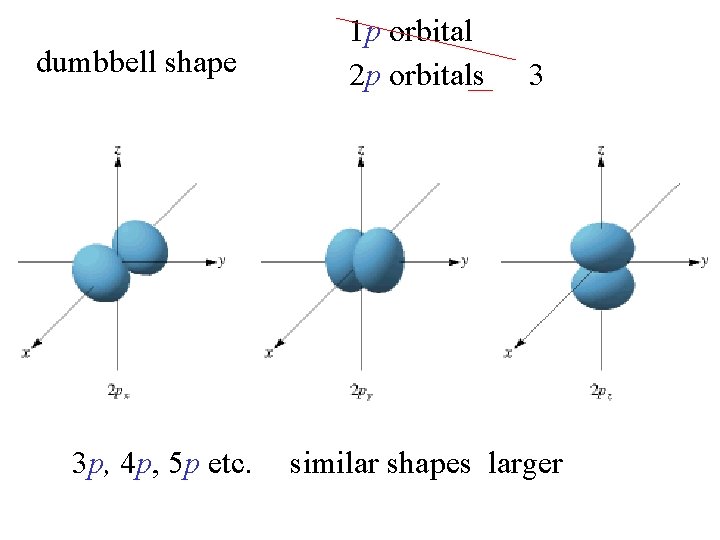
dumbbell shape 3 p, 4 p, 5 p etc. 1 p orbital 2 p orbitals 3 similar shapes larger
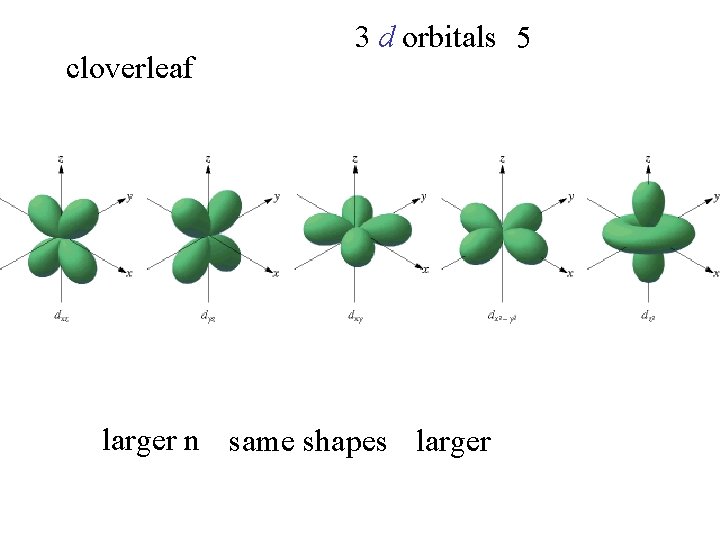
cloverleaf 3 d orbitals 5 larger n same shapes larger
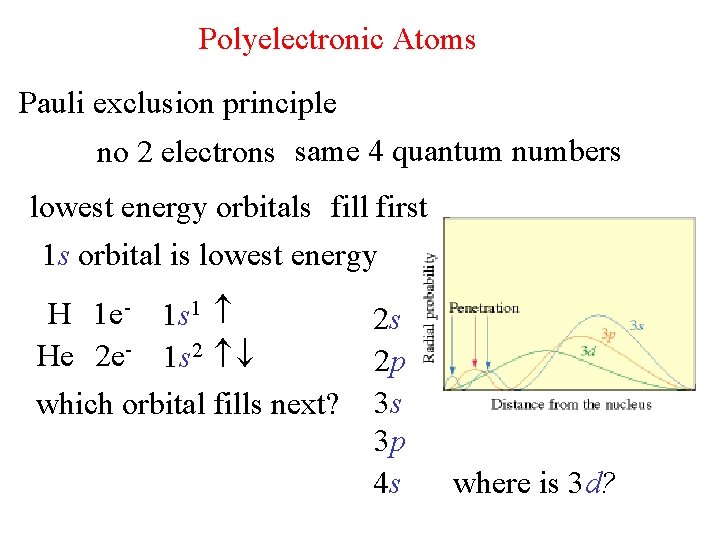
Polyelectronic Atoms Pauli exclusion principle no 2 electrons same 4 quantum numbers lowest energy orbitals fill first 1 s orbital is lowest energy H 1 e- 1 s 1 He 2 e- 1 s 2 which orbital fills next? 2 s 2 p 3 s 3 p 4 s where is 3 d?
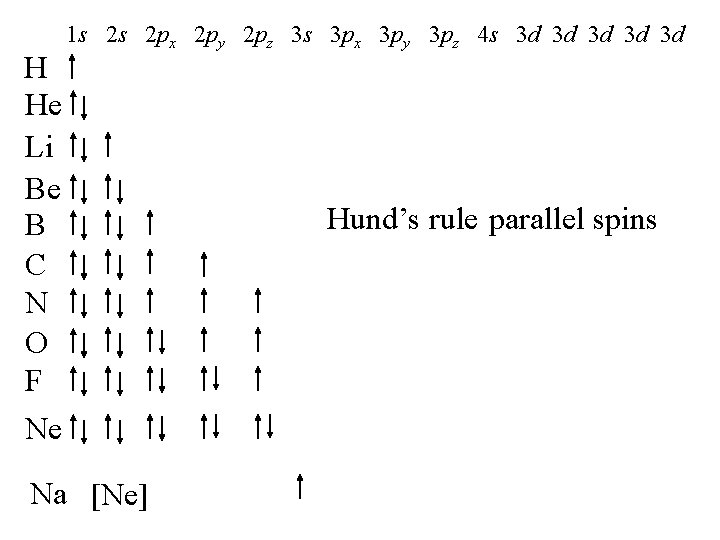
H He Li Be B C N O F Ne 1 s 2 s 2 px 2 py 2 pz 3 s 3 px 3 py 3 pz 4 s 3 d 3 d 3 d Na [Ne] Hund’s rule parallel spins
![K [Ar] Ca [Ar] Sc [Ar] Ti [Ar] V [Ar] 4 s 3 dxz K [Ar] Ca [Ar] Sc [Ar] Ti [Ar] V [Ar] 4 s 3 dxz](http://slidetodoc.com/presentation_image_h/d2855887146e7486eec8bbec77084a99/image-14.jpg)
K [Ar] Ca [Ar] Sc [Ar] Ti [Ar] V [Ar] 4 s 3 dxz 3 dyz 3 dxy 3 dx 2 -z 2 3 dz 2 4 px half full shell stable Cr [Ar] Mn [Ar] full shell stable Cu [Ar]
- Slides: 14