QUANTUM MECHANICAL MODEL OF THE ATOM DEFINITION OF









- Slides: 13

QUANTUM MECHANICAL MODEL OF THE ATOM

DEFINITION OF TERMS 1. 2. 3. QUANTUM – UNIT OF ENERGY ORBITALS – space around a nucleus where the probability of finding an electron is greatest. QUANTUM NUMBERS – set of four numbers used to describe the electron’s energy level in terms of: a. distance from the nucleus b. shape of the orbital c. orientation in space (x, y, z) d. direction of electron spin (clockwise, counter-

ENERGY LEVELS OF THE ATOMIC ORBITALS 1. MAIN ENERGY LEVEL (MEL). - represents the average distance of the electrons from the nucleus. - values 1, 2, 3, 4, 5, 6 and 7

Electrons in MEL closer to the nucleus have the lowest energy

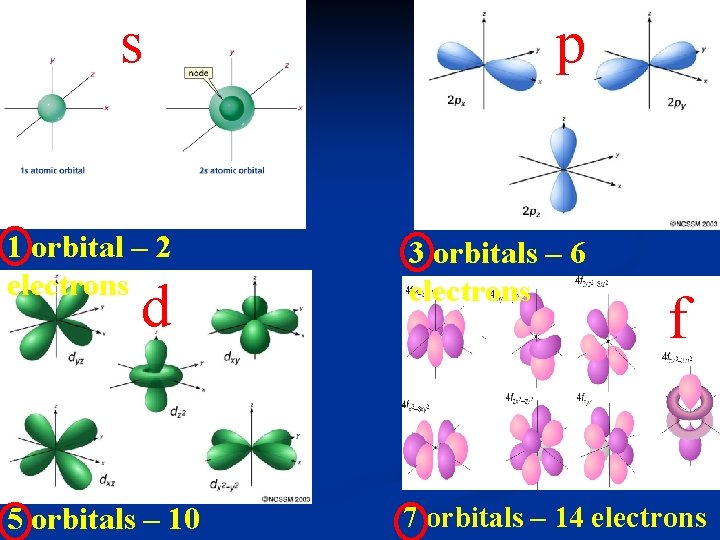
2. SUBLEVELS or SUBSHELLS - define the shapes of the orbitals - has four possible shapes called EACHs, SUBLEVEL p, d and f HAS DIFFERENT NUMBERS OF ORBITALS AND EACH ORBITAL CAN ONLY HAVE A MAXIMUM OF 2 ELECTRONS

s p 1 orbital – 2 electrons 3 orbitals – 6 electrons 5 orbitals – 10 7 orbitals – 14 electrons d f

SUMMARY OF ENERGY LEVELS, ORBITALS AND ELECTRON RELATIONSHIPS MEL (n) MAXIMUM NUMBER OF ORBITALS (n 2) MAXIMUM NUMBER OF ELECTRONS PER ENERGY LEVEL (2 n 2) 1 1 2 2 4 8 3 9 18 4 16 32

ELECTRONIC CONFIGURATION - Process of arranging the electrons around the nucleus The problem? The solution!

THE RESULT!!!

1. 2. 3. RULES GOVERNING ELECTRON CONFIGURATION AUFBAU PRINCIPLE – electrons enter orbitals of lowest energy first. PAULI’S EXCLUSION PRINCIPLE – only two electrons can occupy a single orbital. HUND’S RULE OF MAXIMUM MULTIPLICITY– within the same sublevel, electrons prefer to

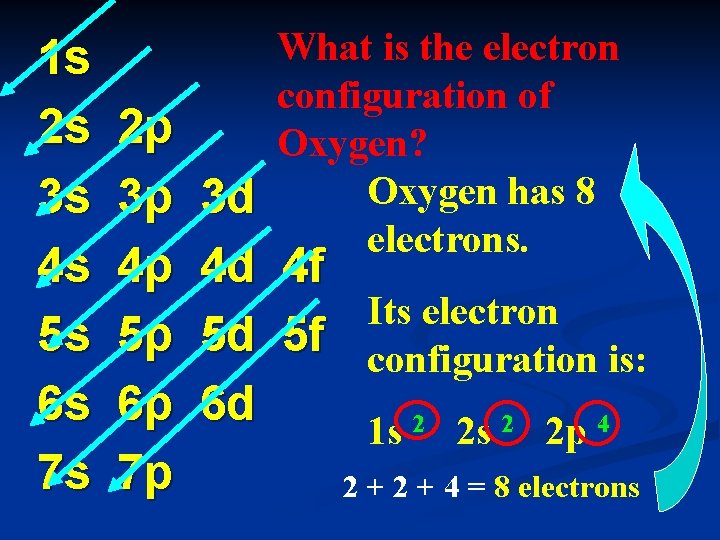
1 s 2 s 3 s 4 s 5 s 6 s 7 s 2 p 3 p 4 p 5 p 6 p 7 p What is the electron configuration of Oxygen? Oxygen has 8 electrons. 3 d 4 d 4 f Its electron 5 d 5 f configuration is: 6 d 1 s 2 2 p 4 2 + 4 = 8 electrons

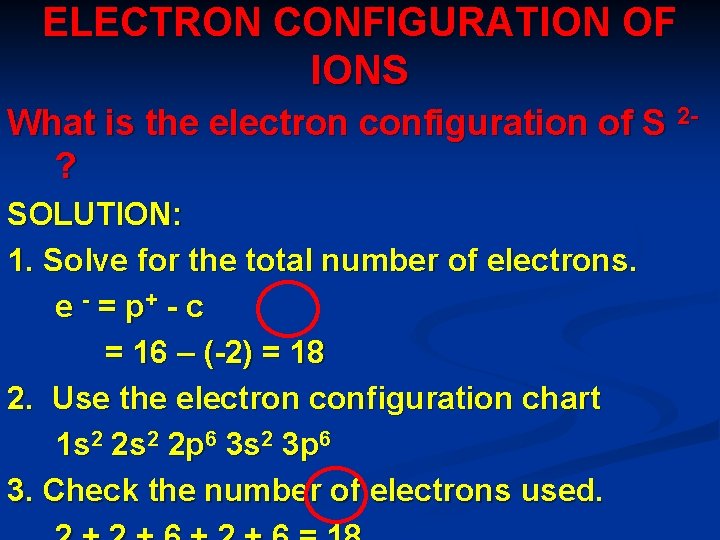
ELECTRON CONFIGURATION OF IONS What is the electron configuration of S 2? SOLUTION: 1. Solve for the total number of electrons. e - = p+ - c = 16 – (-2) = 18 2. Use the electron configuration chart 1 s 2 2 p 6 3 s 2 3 p 6 3. Check the number of electrons used.

EXCEPTIONS TO THE RULE 24 Cr 4 3 d 2 1 s 2 2 s 6 2 p 2 3 s 6 2 3 p 4 s 2 1 s 2 2 s 6 2 p 2 3 s 6 1 3 p 4 s √ 5 3 d 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 √ 9 Cu 1 s 3 d 29 1 s 2 2 p 6 3 s 2 3 p 6 4 s 1 3 d 10