QUANTUM MECHANICAL MODEL OF THE ATOM DEFINITION OF















- Slides: 26

QUANTUM MECHANICAL MODEL OF THE ATOM

DEFINITION OF TERMS 1. 2. 3. QUANTUM – UNIT OF ENERGY ORBITALS – space around a nucleus where the probability of finding an electron is greatest. QUANTUM NUMBERS – set of four numbers used to describe the electron’s energy level in terms of: a. distance from the nucleus b. shape of the orbital c. orientation in space (x, y, z) d. direction of electron spin (clockwise, counter-

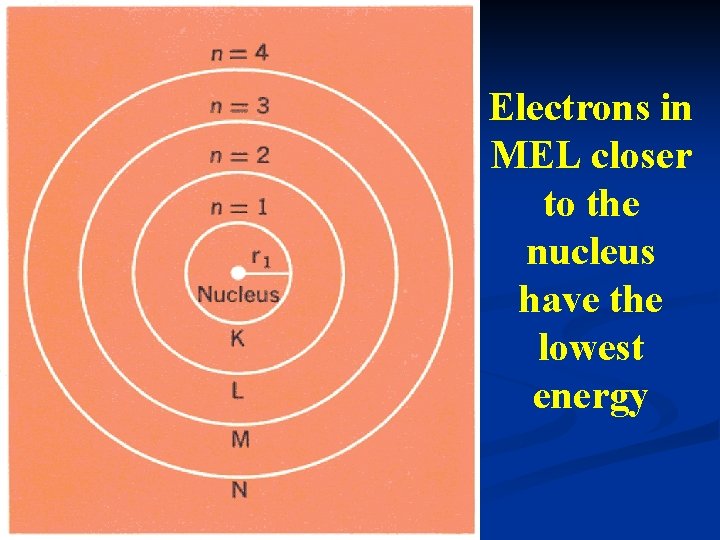
ENERGY LEVELS OF THE ATOMIC ORBITALS 1. MAIN ENERGY LEVEL (MEL). - represents the average distance of the electrons from the nucleus. - values 1, 2, 3, 4, 5, 6 and 7

Electrons in MEL closer to the nucleus have the lowest energy

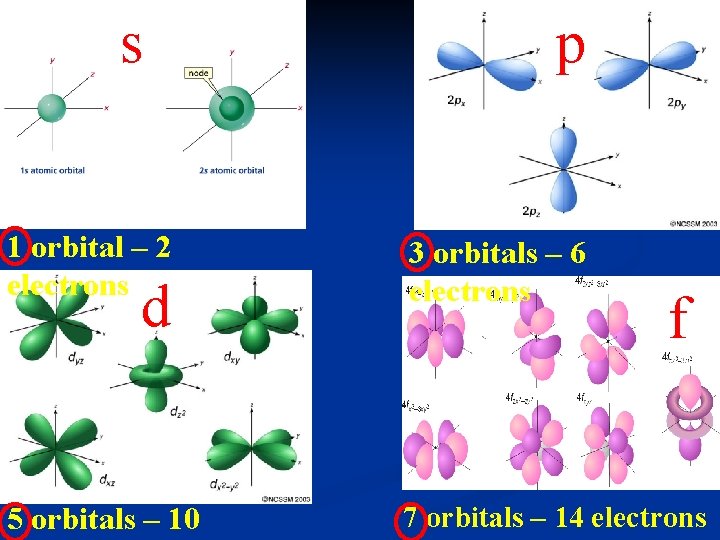
2. SUBLEVELS or SUBSHELLS - define the shapes of the orbitals - has four possible shapes called EACHs, SUBLEVEL p, d and f HAS DIFFERENT NUMBERS OF ORBITALS AND EACH ORBITAL CAN ONLY HAVE A MAXIMUM OF 2 ELECTRONS

s p 1 orbital – 2 electrons 3 orbitals – 6 electrons 5 orbitals – 10 7 orbitals – 14 electrons d f

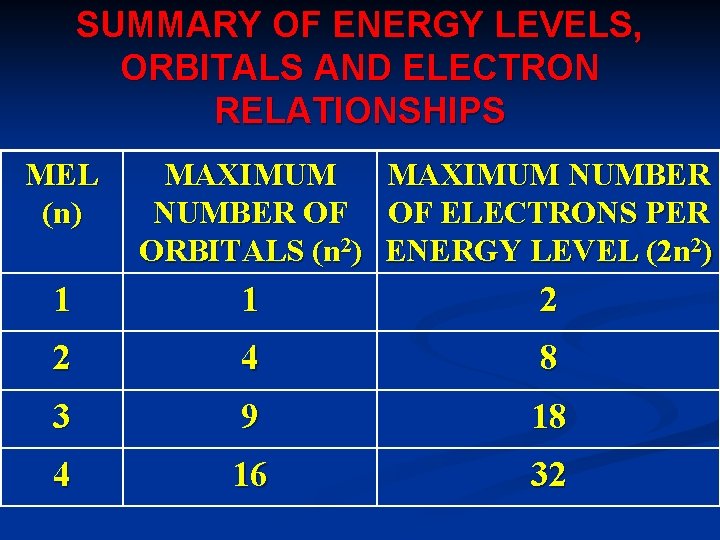
SUMMARY OF ENERGY LEVELS, ORBITALS AND ELECTRON RELATIONSHIPS MEL (n) MAXIMUM NUMBER OF ORBITALS (n 2) MAXIMUM NUMBER OF ELECTRONS PER ENERGY LEVEL (2 n 2) 1 1 2 2 4 8 3 9 18 4 16 32

ELECTRONIC CONFIGURATION - Process of arranging the electrons around the nucleus The problem? The solution!

THE RESULT!!!

1. 2. 3. RULES GOVERNING ELECTRON CONFIGURATION AUFBAU PRINCIPLE – electrons enter orbitals of lowest energy first. PAULI’S EXCLUSION PRINCIPLE – only two electrons can occupy a single orbital. HUND’S RULE OF MAXIMUM MULTIPLICITY– within the same sublevel, electrons prefer to

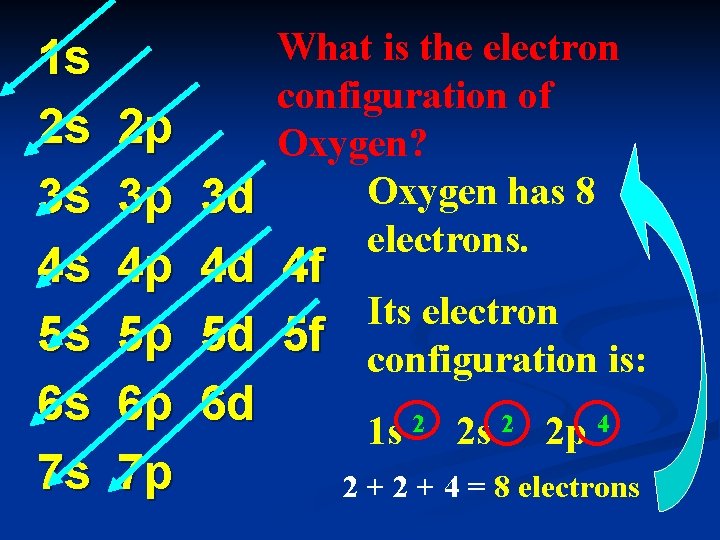
1 s 2 s 3 s 4 s 5 s 6 s 7 s 2 p 3 p 4 p 5 p 6 p 7 p What is the electron configuration of Oxygen? Oxygen has 8 electrons. 3 d 4 d 4 f Its electron 5 d 5 f configuration is: 6 d 1 s 2 2 p 4 2 + 4 = 8 electrons


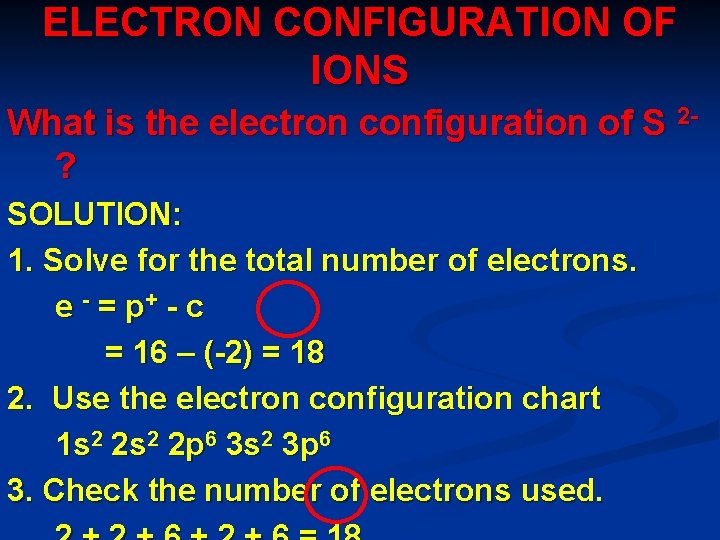
ELECTRON CONFIGURATION OF IONS What is the electron configuration of S 2? SOLUTION: 1. Solve for the total number of electrons. e - = p+ - c = 16 – (-2) = 18 2. Use the electron configuration chart 1 s 2 2 p 6 3 s 2 3 p 6 3. Check the number of electrons used.

EXCEPTIONS TO THE RULE 24 Cr 4 3 d 2 1 s 2 2 s 6 2 p 2 3 s 6 2 3 p 4 s 2 1 s 2 2 s 6 2 p 2 3 s 6 1 3 p 4 s √ 5 3 d 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 √ 9 Cu 1 s 3 d 29 1 s 2 2 p 6 3 s 2 3 p 6 4 s 1 3 d 10

Electron Population Distribution - Shows how the electrons are distributed among the different orbitals - Based on the electron configuration of the elements

Electron Population Distribution Show the electron population distribution for 15 P. P has 15 electrons. Its electron configuration is 1 s 2 2 p 6 3 s 2 3 p 3. The electron population distribution for P is: 1 s 2 s 2 p 3 s 3 p

QUANTUM NUMBERS a. b. c. d. Set of 4 numbers used to describe the electrons in terms of : Distance from the nucleus Shape of the orbitals Orientation in space Direction of electron spin

PRINCIPAL QUANTUM NUMBER, n - - Refers to the main energy levels Related to the average distance of the electron from the nucleus Can only have integral values of n = 1, 2, 3, 4 etc.

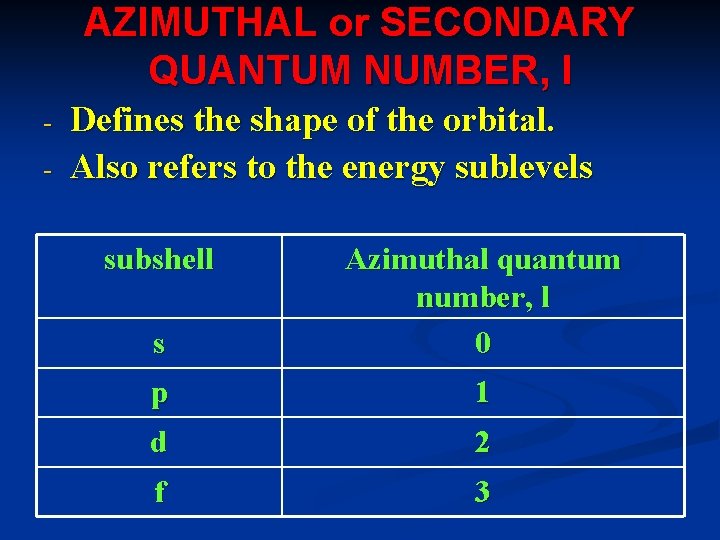
AZIMUTHAL or SECONDARY QUANTUM NUMBER, l - Defines the shape of the orbital. Also refers to the energy sublevels subshell s Azimuthal quantum number, l 0 p 1 d 2 f 3

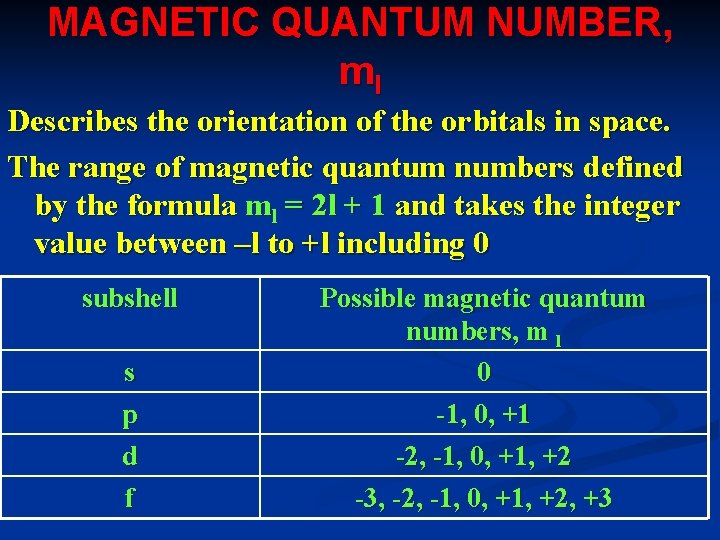
MAGNETIC QUANTUM NUMBER, ml Describes the orientation of the orbitals in space. The range of magnetic quantum numbers defined by the formula ml = 2 l + 1 and takes the integer value between –l to +l including 0 subshell s p d f Possible magnetic quantum numbers, m l 0 -1, 0, +1 -2, -1, 0, +1, +2 -3, -2, -1, 0, +1, +2, +3

SPIN QUANTUM NUMBER, ms - differentiates how two electrons behave under a magnetic field. - Can only have two possible values +½ and -½

Example. What are the possible quantum numbers for the outermost electron of oxygen? SOLUTION: Identify the final orbital occupied by the electron. 1 s 2 2 p 4 2 p 4 n= 2 l= 1 ml = ms = -1, 0, +1 +½ , -½

What are the possible quantum numbers for the outermost electron of chromium? SOLUTION: Identify the final orbital occupied by the electron. 1 s 2 2 p 6 3 s 2 3 p 6 4 s 1 3 d 5 3 d 5 n= 3 l= 2 ml = ms = -2, -1, 0, +1, +2 +½ , -½

For each subshells given below, give the maximum number of orbitals and electrons possible Subshell or sublevel 8 s 3 p 3 d 6 f 6 s 5 d 4 f Number of orbitals Number of electrons


Electron Population Distribution or Electron Orbital Notation Show the electron population distribution for 15 P. P has 15 electrons. Its electron configuration is 1 s 2 2 p 6 3 s 2 3 p 3. The electron population distribution for P is: 1 s 2 s 2 p 3 s 3 p