Quantum Mechanical Model Main Concept The currently accepted









- Slides: 27

Quantum Mechanical Model Main Concept: The currently accepted best model of the atom is based on the quantum mechanical (QM) model.

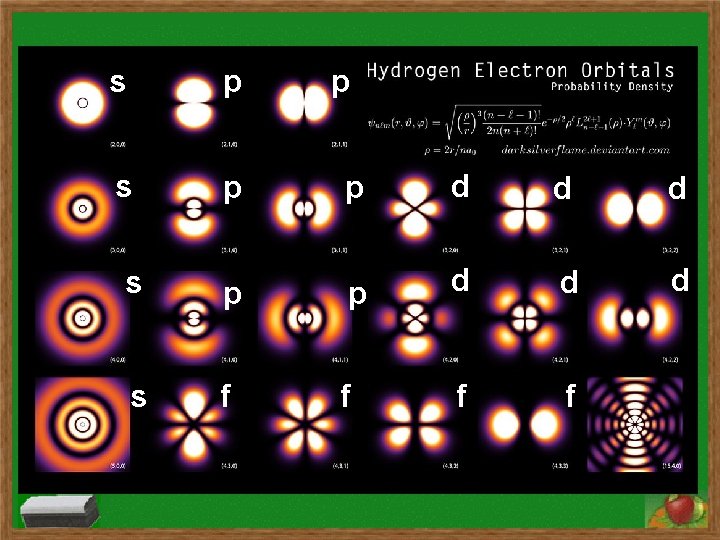
Electrons & QM Model Subshells & Orbitals s p Evidence for Quantum Mechanical Model What we expect without subshells d f What subshells explain Valence Electrons and Electron Configurations

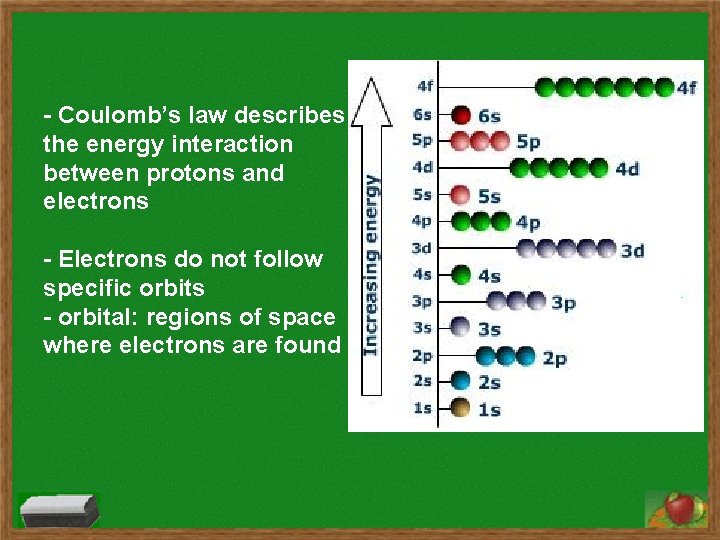
- Coulomb’s law describes the energy interaction between protons and electrons - Electrons do not follow specific orbits - orbital: regions of space where electrons are found

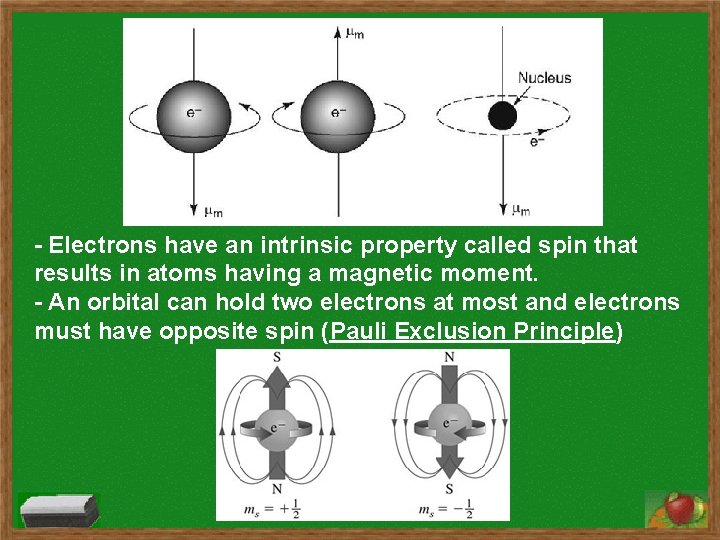
- Electrons have an intrinsic property called spin that results in atoms having a magnetic moment. - An orbital can hold two electrons at most and electrons must have opposite spin (Pauli Exclusion Principle)

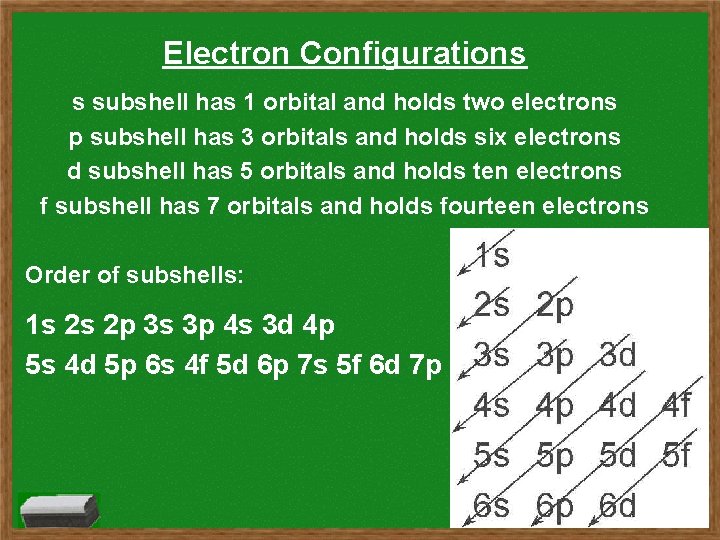
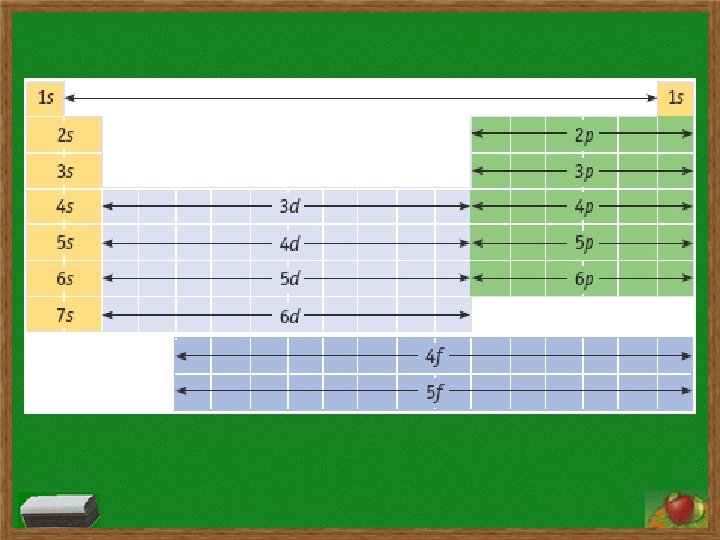
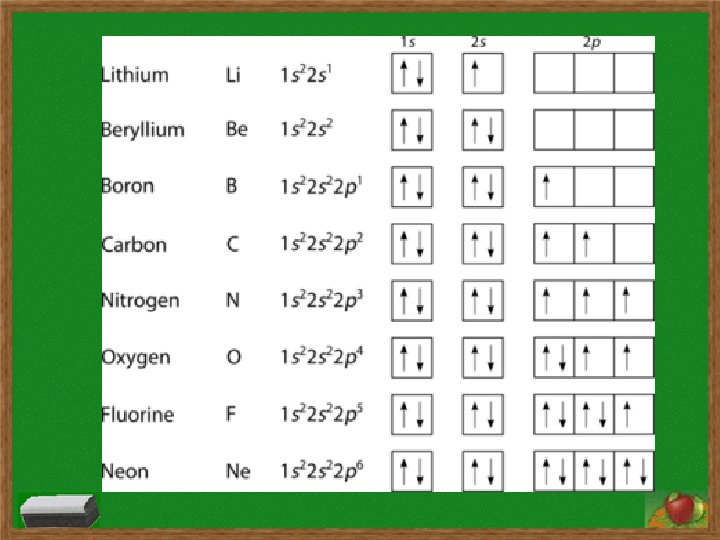
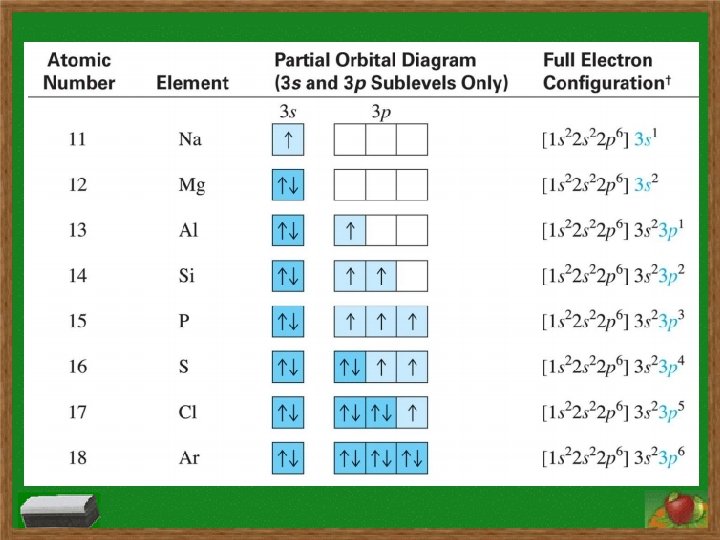
Electron Configurations s subshell has 1 orbital and holds two electrons p subshell has 3 orbitals and holds six electrons d subshell has 5 orbitals and holds ten electrons f subshell has 7 orbitals and holds fourteen electrons Order of subshells: 1 s 2 s 2 p 3 s 3 p 4 s 3 d 4 p 5 s 4 d 5 p 6 s 4 f 5 d 6 p 7 s 5 f 6 d 7 p

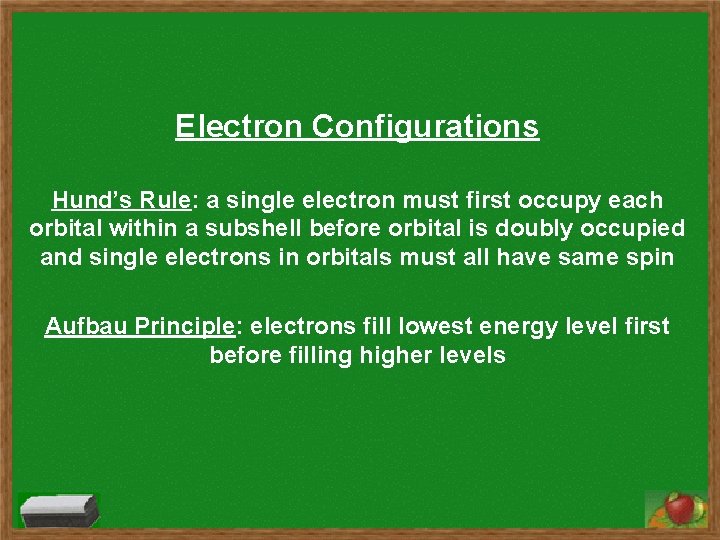
Electron Configurations Hund’s Rule: a single electron must first occupy each orbital within a subshell before orbital is doubly occupied and single electrons in orbitals must all have same spin Aufbau Principle: electrons fill lowest energy level first before filling higher levels

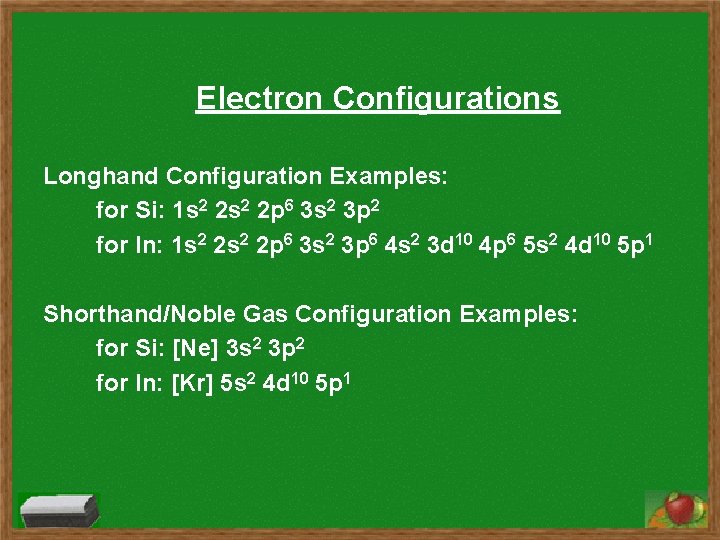
Electron Configurations Longhand Configuration Examples: for Si: 1 s 2 2 p 6 3 s 2 3 p 2 for In: 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 6 5 s 2 4 d 10 5 p 1 Shorthand/Noble Gas Configuration Examples: for Si: [Ne] 3 s 2 3 p 2 for In: [Kr] 5 s 2 4 d 10 5 p 1

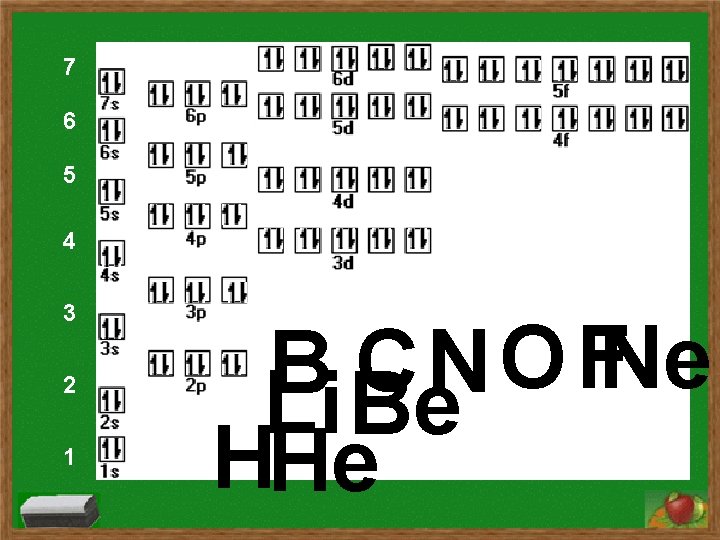
7 6 5 4 3 2 1 O FNe CN B Li Be HHe

Group 1 A: H: 1 s 1 Li: 1 s 2 2 s 1 2 2 6 1 Na: 1 s 2 s 2 p 3 s K: 1 s 2 2 p 6 3 s 2 3 p 6 4 s 1

Group 2 A: Be: 1 s 2 2 s 2 Mg: 1 s 2 2 p 6 3 s 2 Ca: 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2

Group 3 A: B: 1 s 2 2 p 1 Al: 1 s 2 2 p 6 3 s 2 3 p 1 Ga: 1 s 2 2 p 6 3 s 2 3 p 1 4 s 2 3 d 10 4 p 1 Ga: 1 s 2 2 p 6 3 s 2 3 p 1 3 d 10 4 s 2 4 p 1

Group 4 A: C: 1 s 2 2 p 2 2 2 6 2 2 Si: 1 s 2 s 2 p 3 s 3 p Ge: 1 s 2 2 p 6 3 s 2 3 p 1 4 s 2 3 d 10 4 p 2 Ge: 1 s 2 2 p 6 3 s 2 3 p 1 3 d 10 4 s 2 4 p 2

Group 5 A: N: 1 s 2 2 p 3 P: 1 s 2 2 p 6 3 s 2 3 p 3 As: 1 s 2 2 p 6 3 s 2 3 p 1 4 s 2 3 d 10 4 p 3 As: 1 s 2 2 p 6 3 s 2 3 p 1 3 d 10 4 s 2 4 p 3

Group 6 A: O: 1 s 2 2 p 4 S: 1 s 2 2 p 6 3 s 2 3 p 4 Se: 1 s 2 2 p 6 3 s 2 3 p 1 4 s 2 3 d 10 4 p 4 Se: 1 s 2 2 p 6 3 s 2 3 p 1 3 d 10 4 s 2 4 p 4

Group 7 A: F: 1 s 2 2 p 5 Cl: 1 s 2 2 p 6 3 s 2 3 p 5 Br: 1 s 2 2 p 6 3 s 2 3 p 1 4 s 2 3 d 10 4 p 5 Br: 1 s 2 2 p 6 3 s 2 3 p 1 3 d 10 4 s 2 4 p 5

Group 8 A: 2 1 s He: Ne: 1 s 2 2 p 6 Ar: 1 s 2 2 p 6 3 s 2 3 p 6 Kr: 1 s 2 2 p 6 3 s 2 3 p 1 4 s 2 3 d 10 4 p 6 Kr: 1 s 2 2 p 6 3 s 2 3 p 1 3 d 10 4 s 2 4 p 6

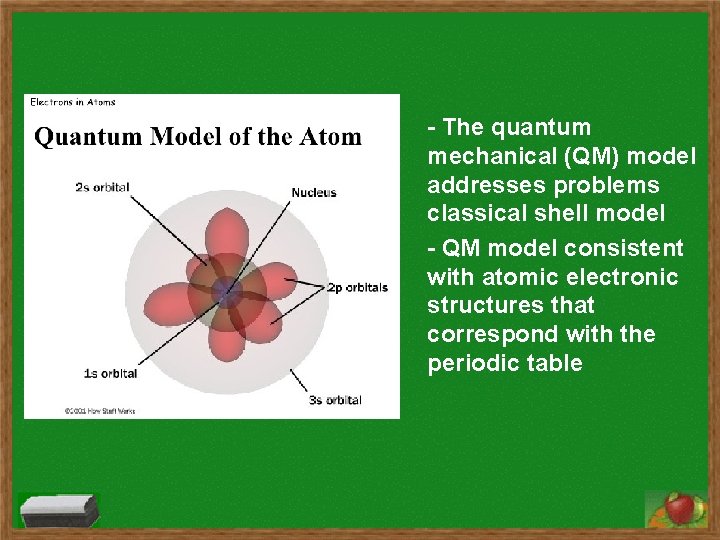
- The quantum mechanical (QM) model addresses problems classical shell model - QM model consistent with atomic electronic structures that correspond with the periodic table

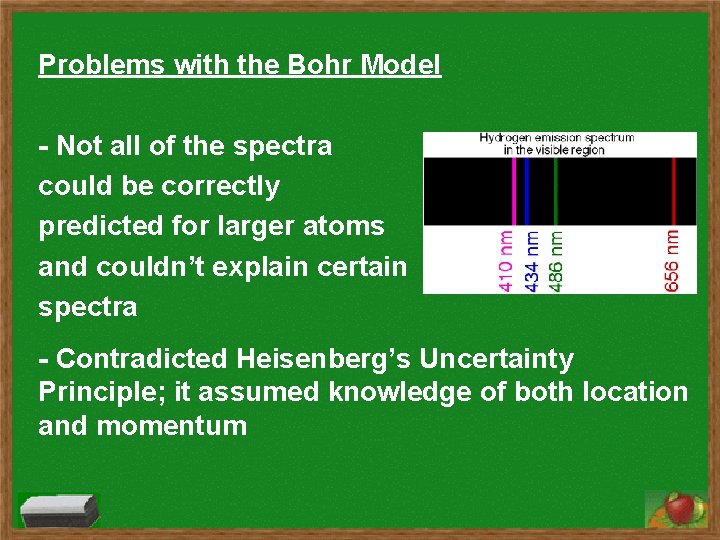
Problems with the Bohr Model - Not all of the spectra could be correctly predicted for larger atoms and couldn’t explain certain spectra - Contradicted Heisenberg’s Uncertainty Principle; it assumed knowledge of both location and momentum

What does the QM model address? - The dual nature of the electron (as a particle and a wave) - Correctly predicts spectra - Allows for probability waves in agreement with Heisenberg’s Uncertainty Principle - Consistent with periodic models, data, and trends


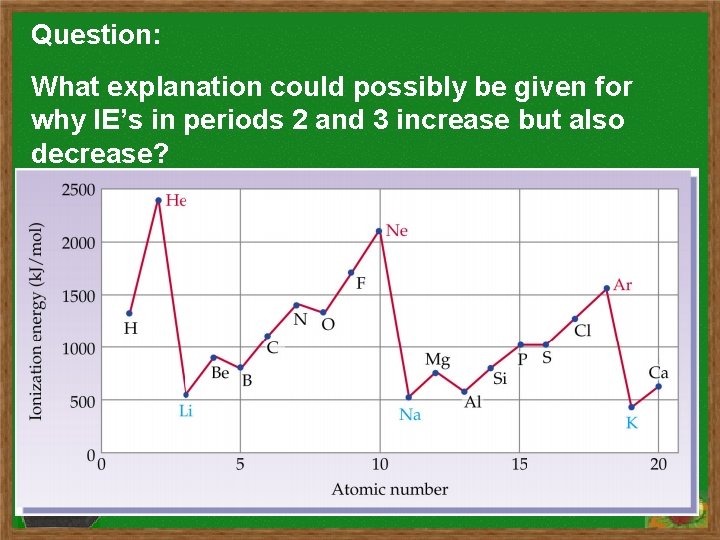
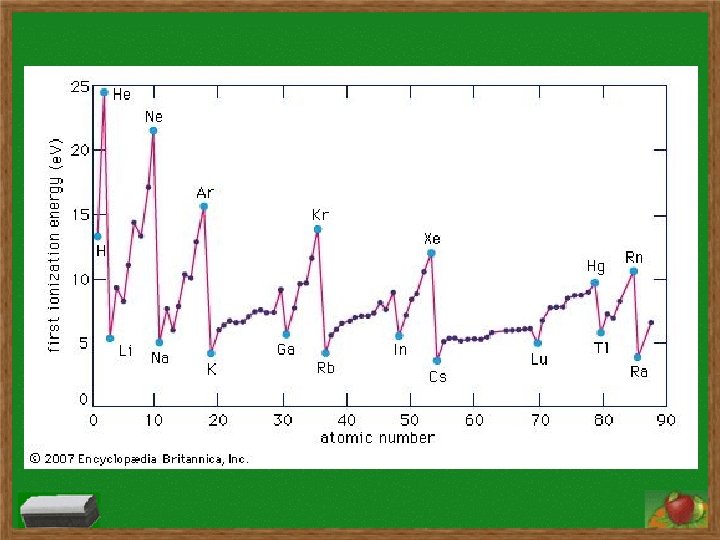
Question: What explanation could possibly be given for why IE’s in periods 2 and 3 increase but also decrease?




- The QM model can be appropriately solved using computers and serves as the basis for software that calculates the structure and reactivity of molecules.

s p p p d d d f s p p d s f f f
