Quantum Electron Configurations Erwin Schrdinger 1887 1961 The




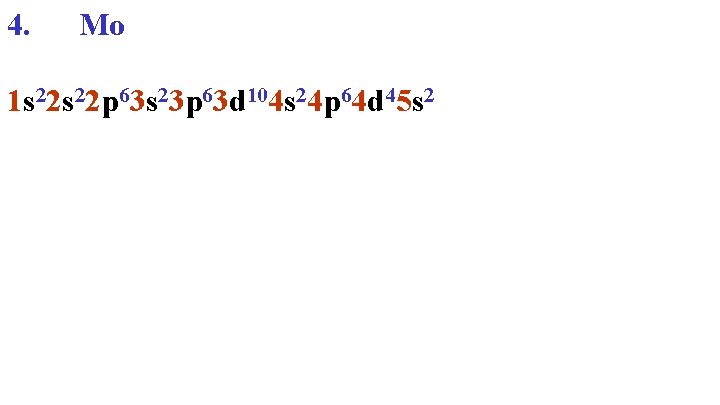

- Slides: 32

Quantum Electron Configurations

Erwin Schrödinger 1887 - 1961

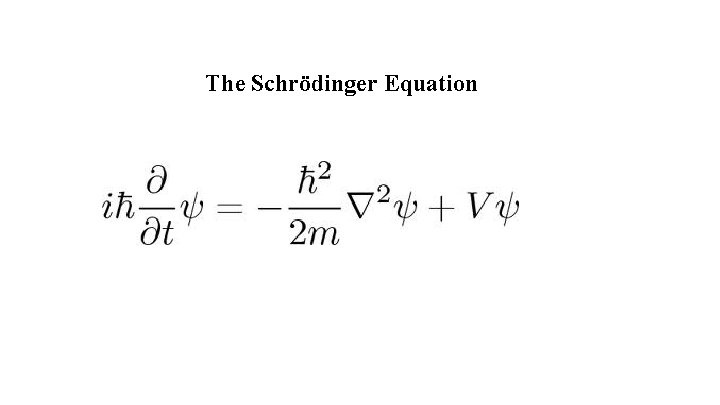
The Schrödinger Equation

Werner Heisenberg 1901 – 1976

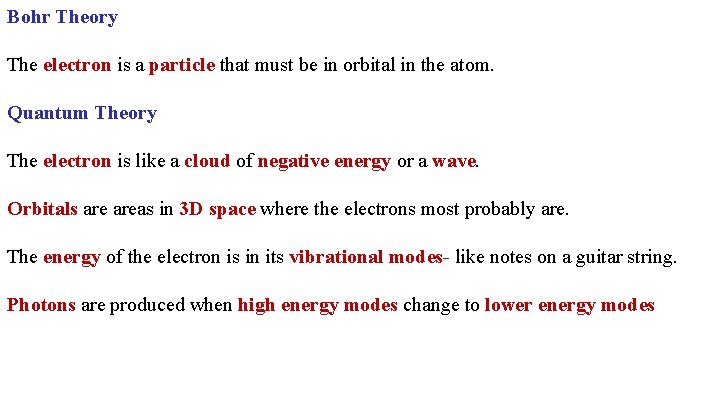
Bohr Theory The electron is a particle that must be in orbital in the atom. Quantum Theory The electron is like a cloud of negative energy or a wave. Orbitals areas in 3 D space where the electrons most probably are. The energy of the electron is in its vibrational modes- like notes on a guitar string. Photons are produced when high energy modes change to lower energy modes

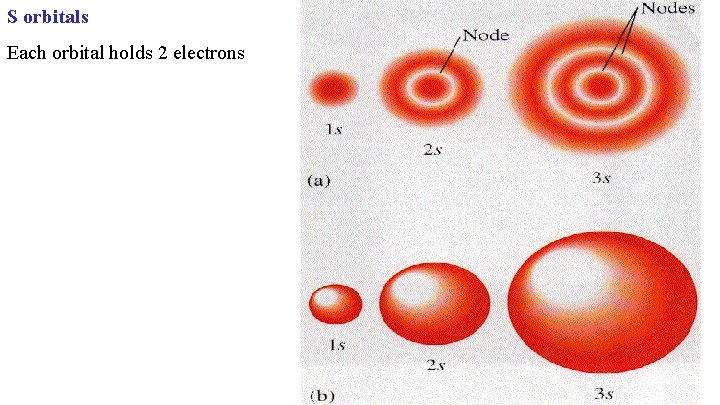
S orbitals Each orbital holds 2 electrons

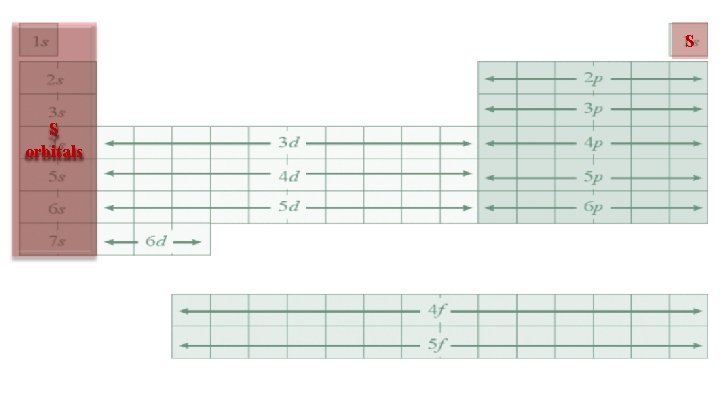
S S orbitals

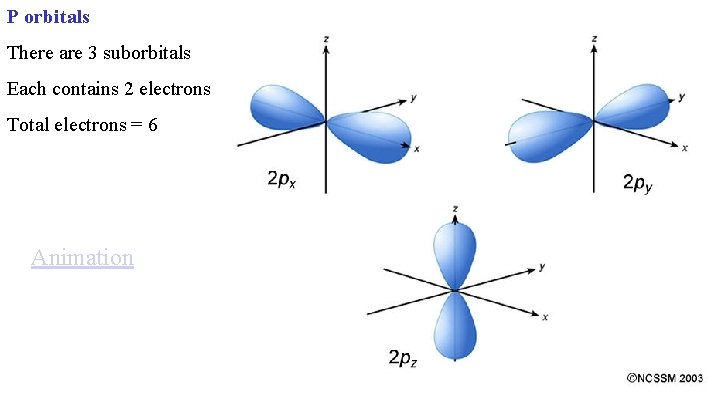
P orbitals There are 3 suborbitals Each contains 2 electrons Total electrons = 6 Animation

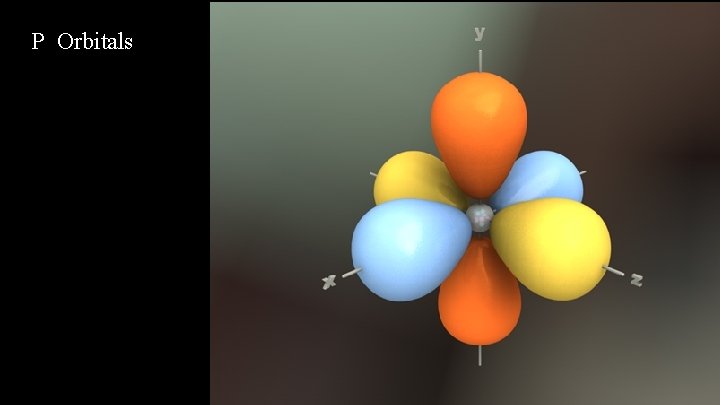
P Orbitals

P Orbitals

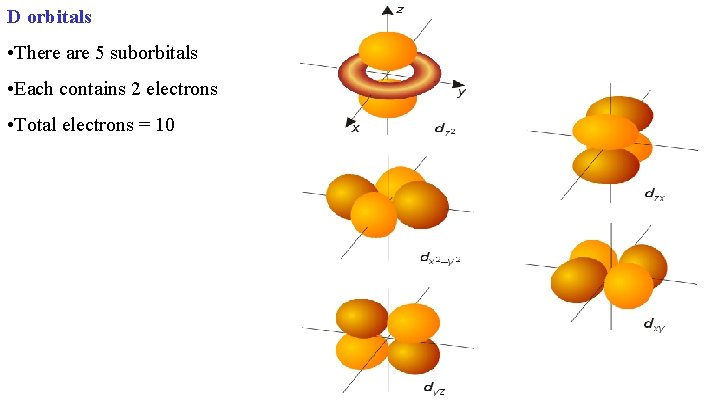
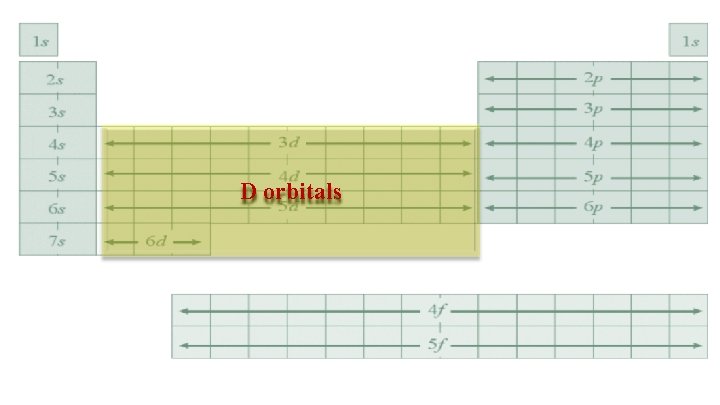
D orbitals • There are 5 suborbitals • Each contains 2 electrons • Total electrons = 10

D orbitals

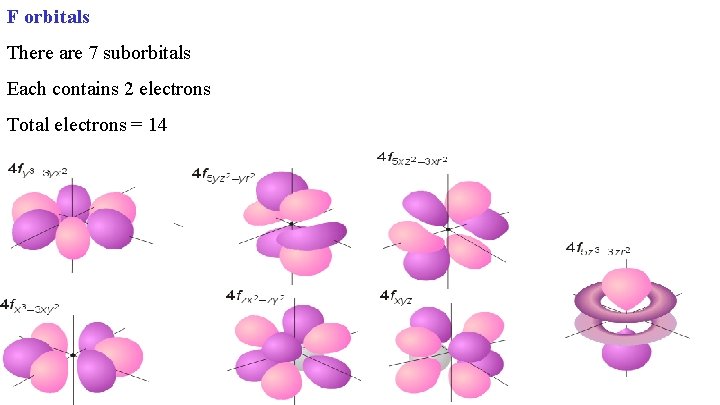
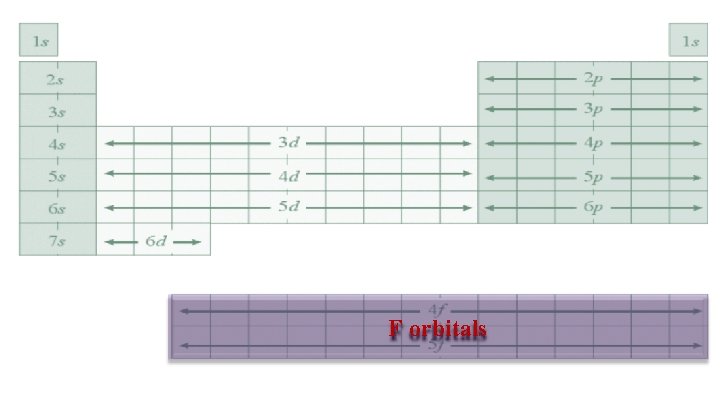
F orbitals There are 7 suborbitals Each contains 2 electrons Total electrons = 14

F orbitals

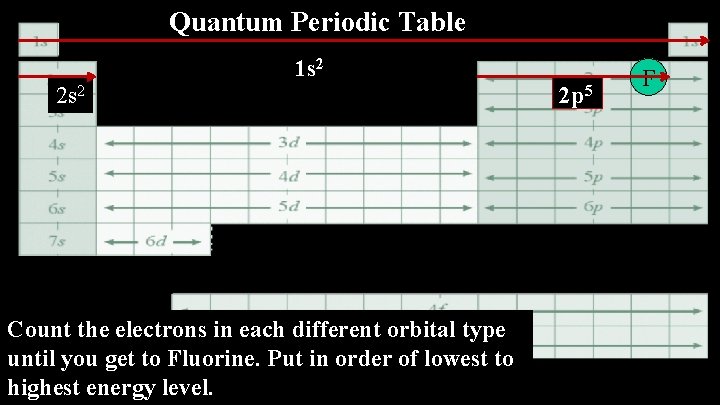
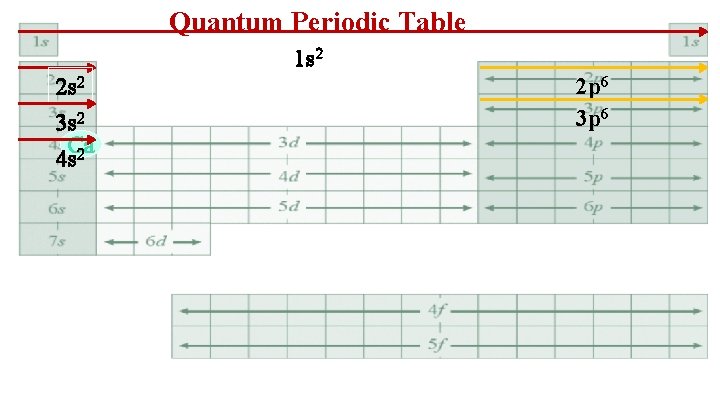
Quantum Periodic Table 1 s 2 2 s 2 Count the electrons in each different orbital type until you get to Fluorine. Put in order of lowest to highest energy level. 2 p 5 F

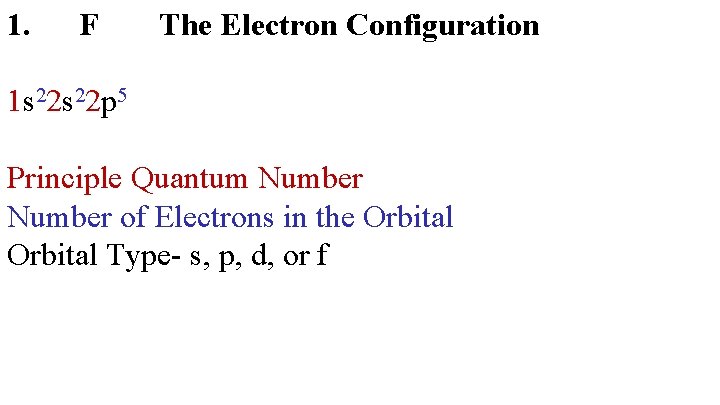
1. F The Electron Configuration 1 s 22 p 5 Principle Quantum Number of Electrons in the Orbital Type- s, p, d, or f

Quantum Periodic Table 1 s 2 2 s 2 3 s 2 Ca 4 s 2 2 p 6 3 p 6

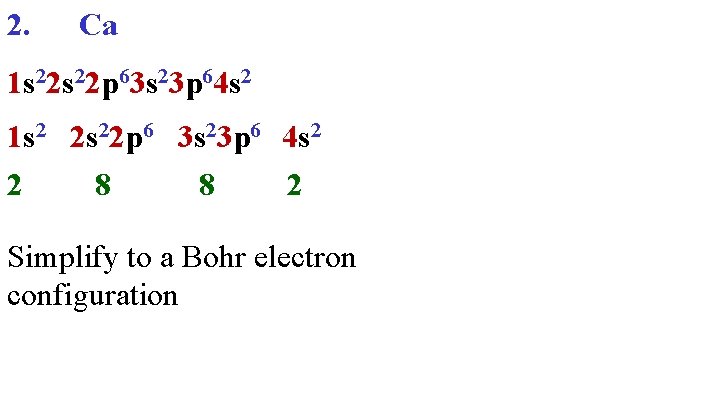
2. Ca 1 s 22 p 63 s 23 p 64 s 2 1 s 2 2 s 22 p 6 3 s 23 p 6 4 s 2 2 8 8 2 Simplify to a Bohr electron configuration

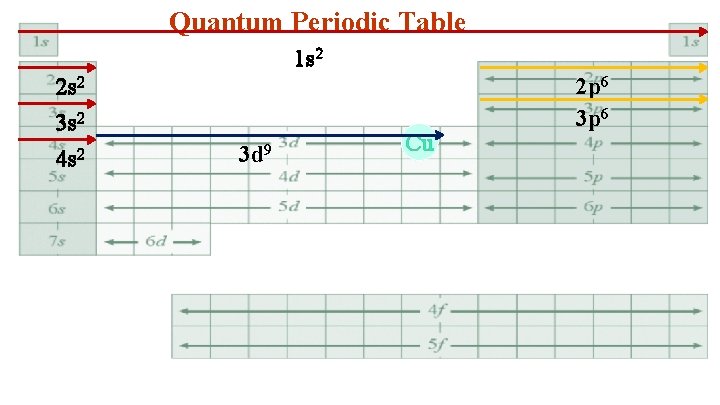
Quantum Periodic Table 1 s 2 2 s 2 3 s 2 4 s 2 3 d 9 Cu 2 p 6 3 p 6

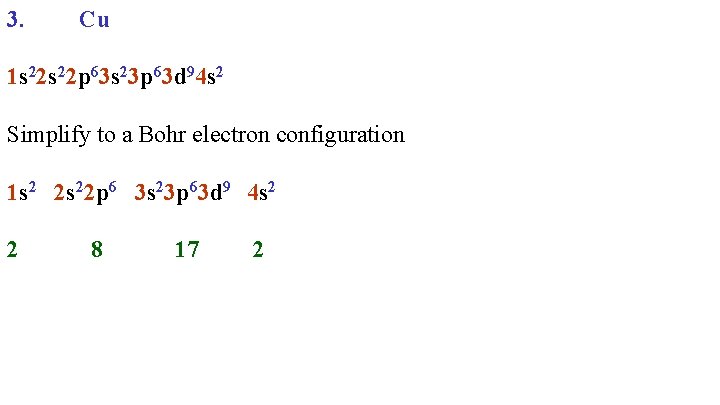
3. Cu 1 s 22 p 63 s 23 p 63 d 94 s 2 Simplify to a Bohr electron configuration 1 s 2 2 s 22 p 6 3 s 23 p 63 d 9 4 s 2 2 8 17 2

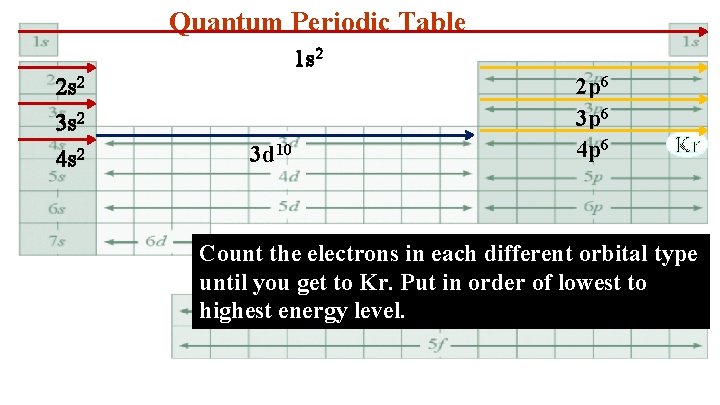
Quantum Periodic Table 1 s 2 2 s 2 3 s 2 4 s 2 3 d 10 2 p 6 3 p 6 4 p 6 Kr Count the electrons in each different orbital type until you get to Kr. Put in order of lowest to highest energy level.

4. Kr 1 s 22 p 63 s 23 p 63 d 104 s 24 p 6 Simplify to a Bohr electron configuration 1 s 2 2 s 22 p 6 3 s 23 p 63 d 10 4 s 24 p 6 2 8 18 8

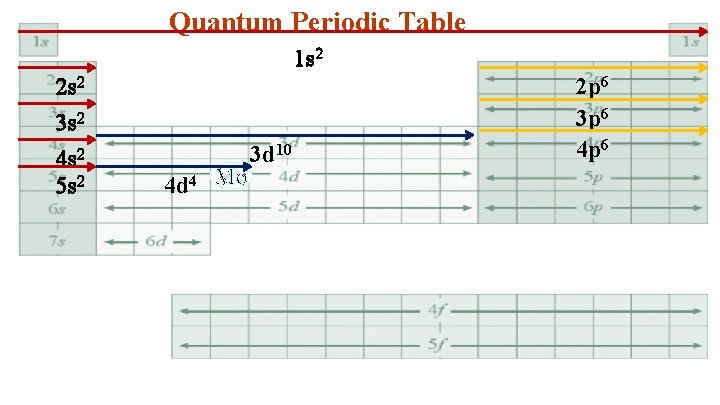
Quantum Periodic Table 1 s 2 2 s 2 3 s 2 4 s 2 5 s 2 4 d 4 Mo 3 d 10 2 p 6 3 p 6 4 p 6
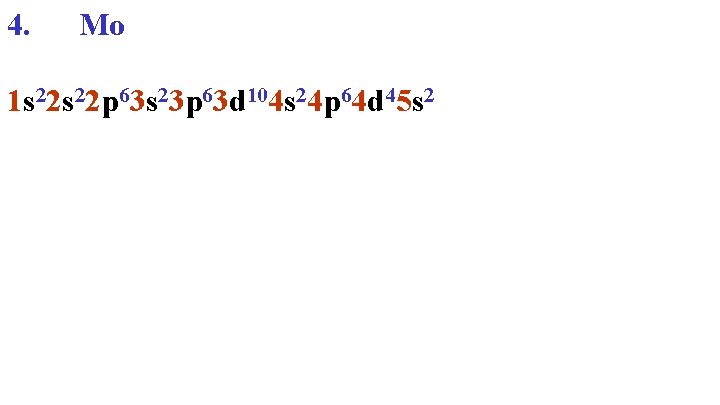
4. Mo 1 s 22 p 63 s 23 p 63 d 104 s 24 p 64 d 45 s 2

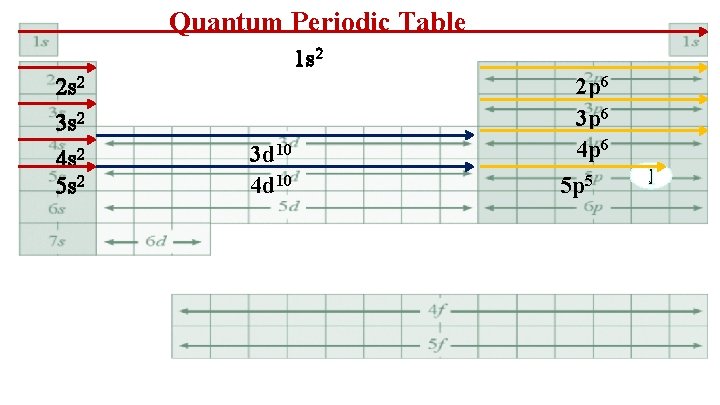
Quantum Periodic Table 1 s 2 2 s 2 3 s 2 4 s 2 5 s 2 3 d 10 4 d 10 2 p 6 3 p 6 4 p 6 5 p 5 I

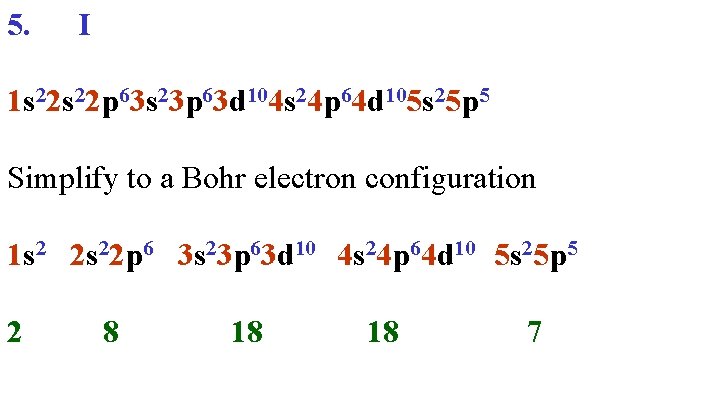
5. I 1 s 22 p 63 s 23 p 63 d 104 s 24 p 64 d 105 s 25 p 5 Simplify to a Bohr electron configuration 1 s 2 2 s 22 p 6 3 s 23 p 63 d 10 4 s 24 p 64 d 10 5 s 25 p 5 2 8 18 18 7

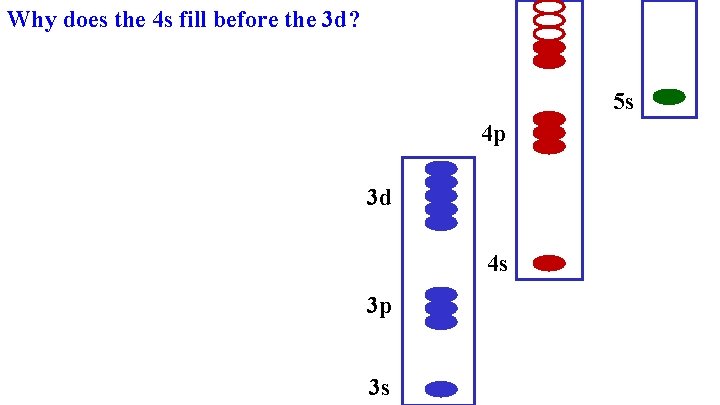
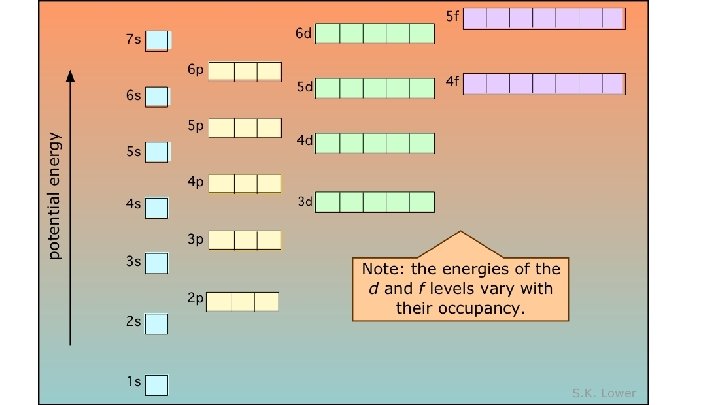
Why does the 4 s fill before the 3 d? 5 s 4 p 3 d 4 s 3 p 3 s


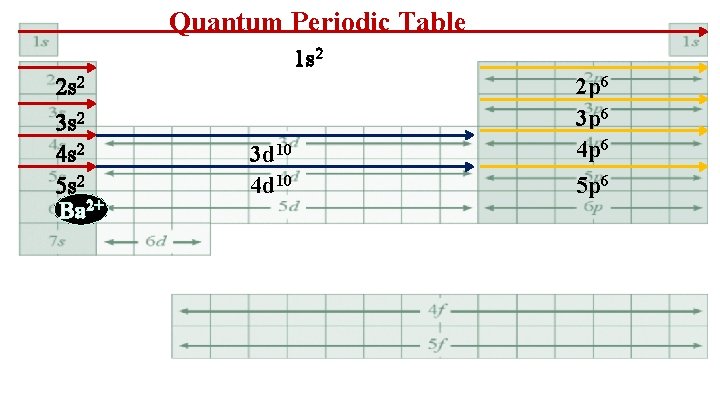
Quantum Periodic Table 1 s 2 2 s 2 3 s 2 4 s 2 5 s 2 Ba 2+ 3 d 10 4 d 10 2 p 6 3 p 6 4 p 6 5 p 6

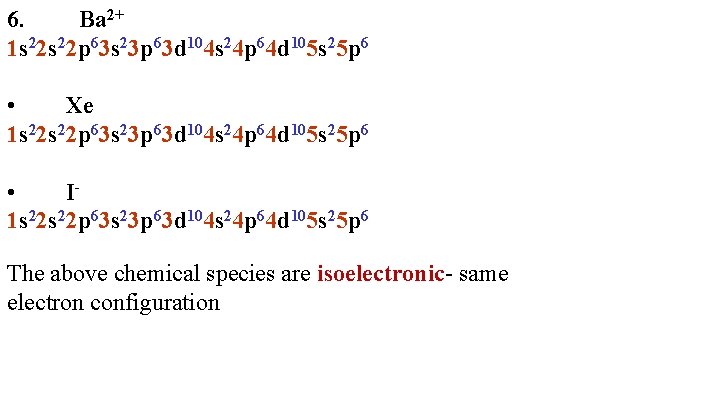
6. Ba 2+ 1 s 22 p 63 s 23 p 63 d 104 s 24 p 64 d 105 s 25 p 6 • Xe 1 s 22 p 63 s 23 p 63 d 104 s 24 p 64 d 105 s 25 p 6 • I 1 s 22 p 63 s 23 p 63 d 104 s 24 p 64 d 105 s 25 p 6 The above chemical species are isoelectronic- same electron configuration


Ishihara Test for Colour Blindness – if you can read all of the numbers you have good colour vision Atomic Theory Song