Quantitative PCR Session 2 Overview of q PCR





![Quantitative PCR • • Reaction efficiency = [10 (-1/m) ]-1 Reaction efficiency of 100% Quantitative PCR • • Reaction efficiency = [10 (-1/m) ]-1 Reaction efficiency of 100%](https://slidetodoc.com/presentation_image/4a426d53c89863c25d974e9c7fcb00d5/image-44.jpg)
- Slides: 44

Quantitative PCR Session 2: Overview of q. PCR Presented by: Robert O'Brien Training Specialist – Forensic Biology

Quantitative PCR Session 2 - Overview of q. PCR Differences between PCR and q. PCR Why Real- Time PCR Mechanism Phases of q. PCR amplification q. PCR chemistry q. PCR process Relationship between the CT value and the quantity of DNA

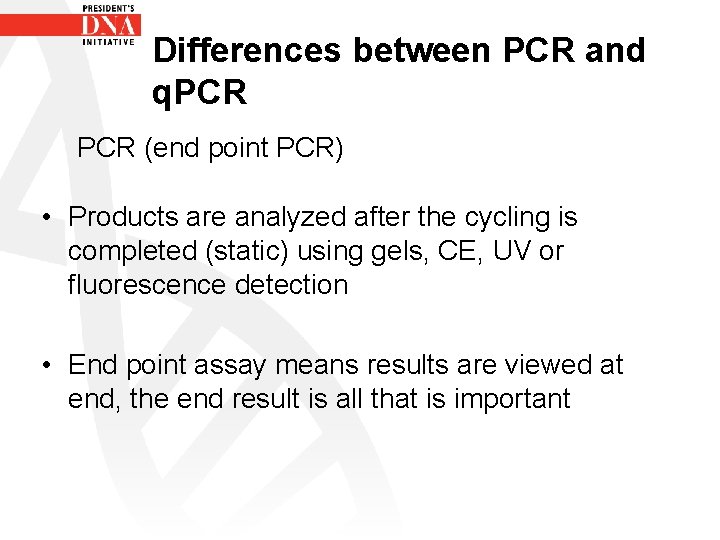
Differences between PCR and q. PCR (end point PCR) • Products are analyzed after the cycling is completed (static) using gels, CE, UV or fluorescence detection • End point assay means results are viewed at end, the end result is all that is important

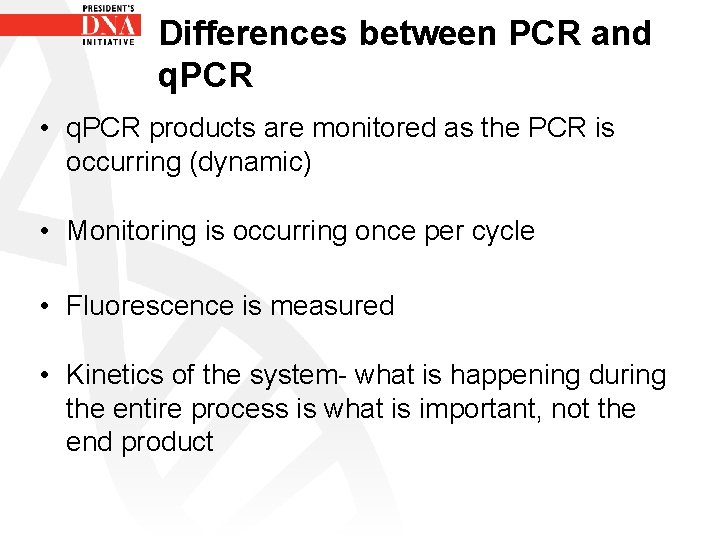
Differences between PCR and q. PCR • q. PCR products are monitored as the PCR is occurring (dynamic) • Monitoring is occurring once per cycle • Fluorescence is measured • Kinetics of the system- what is happening during the entire process is what is important, not the end product

Why Real-Time PCR (Advantages and Disadvantages) – Advantages • Availability of commercial q. PCR kits and instrumentation • Higher throughput and less user intervention • Simple data analysis • Software rapidly analyzes data

Why Real-Time PCR • Advantages • Process sensitive to same inhibitors as end point amplification due to presence of an internal control • No post PCR manipulation • High sensitivity • Large range of quantities that can be detected • Assays can be made to target different types of DNA and in some cases can be multiplexed

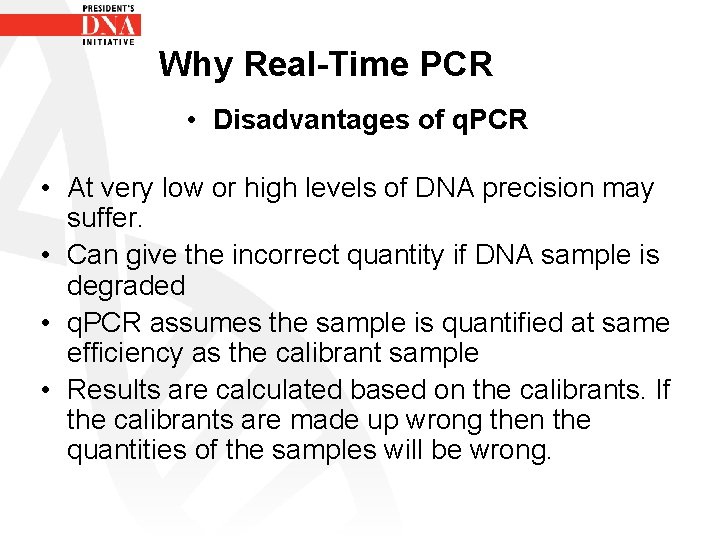
Why Real-Time PCR • Disadvantages of q. PCR • At very low or high levels of DNA precision may suffer. • Can give the incorrect quantity if DNA sample is degraded • q. PCR assumes the sample is quantified at same efficiency as the calibrant sample • Results are calculated based on the calibrants. If the calibrants are made up wrong then the quantities of the samples will be wrong.

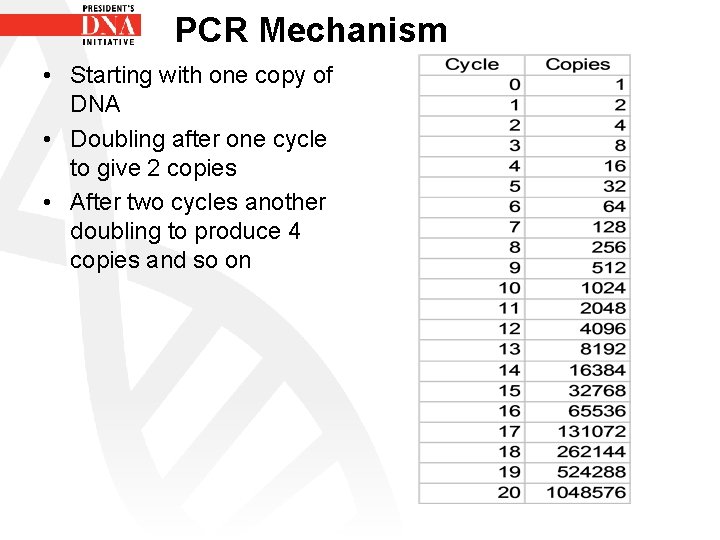
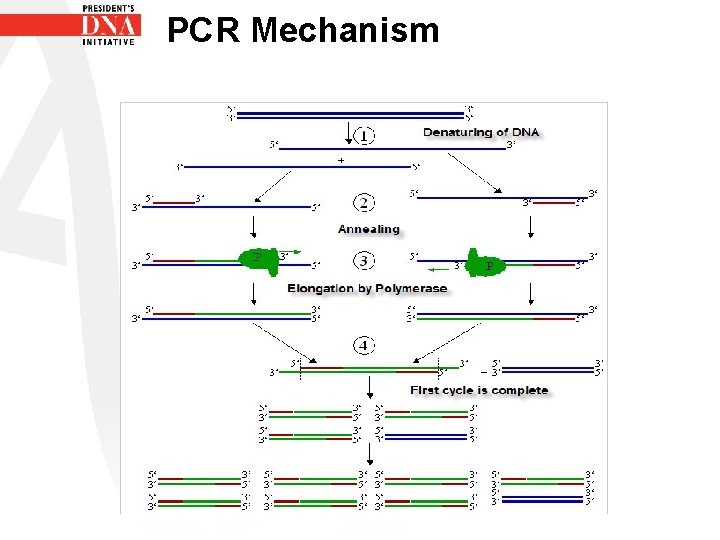
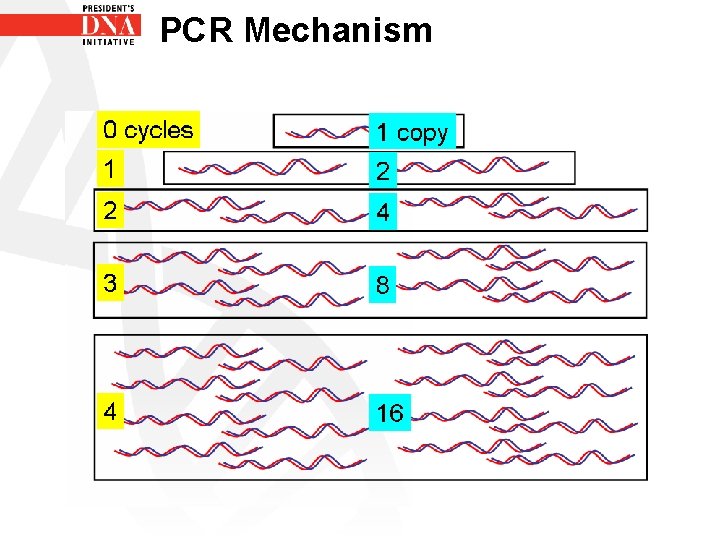
PCR Mechanism • PCR- Polymerase Chain Reaction • Result of the PCR reaction is an exponential increase in PCR products • The amount of DNA present theoretically doubles with every cycle

PCR Mechanism • Starting with one copy of DNA • Doubling after one cycle to give 2 copies • After two cycles another doubling to produce 4 copies and so on

PCR Mechanism

PCR Mechanism

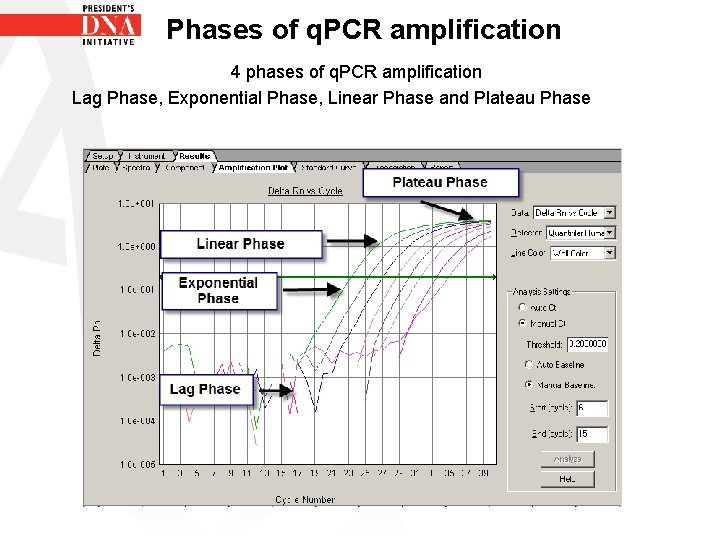
Phases of q. PCR amplification • There are 4 Phases that occur during the PCR process. • • 1 st Phase- Lag phase 2 nd Phase- Exponential phase 3 rd Phase- Linear phase 4 th Phase- Plateau phase

Phases of q. PCR amplification 4 phases of q. PCR amplification Lag Phase, Exponential Phase, Linear Phase and Plateau Phase

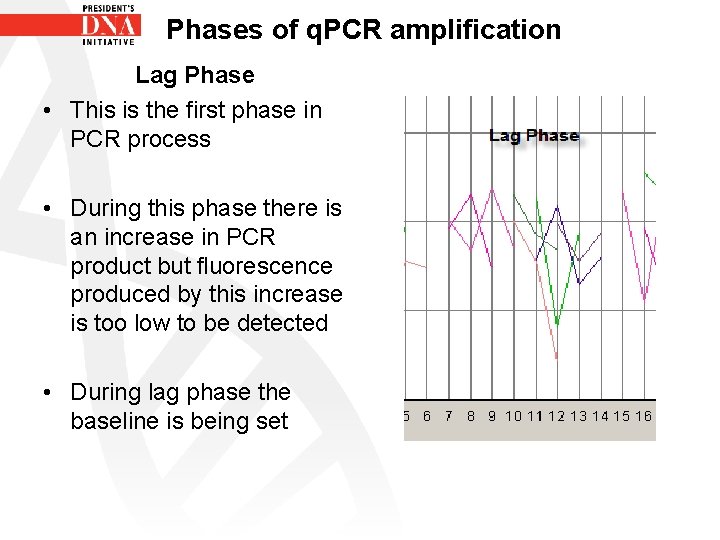
Phases of q. PCR amplification Lag Phase • This is the first phase in PCR process • During this phase there is an increase in PCR product but fluorescence produced by this increase is too low to be detected • During lag phase the baseline is being set

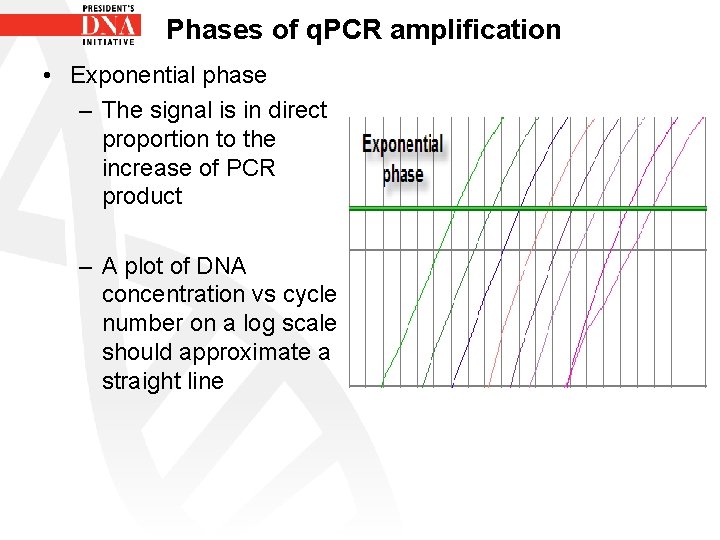
Phases of q. PCR amplification Exponential phase • Also known as the geometric phase • During the exponential phase PCR efficiency is at 100% • There is an abundance of all reaction components - polymerase, free d. NTPS, template DNA, primers etc. • As PCR product continues to increase the ratio of Ampli Taq Gold DNA polymerase to PCR product decreases

Phases of q. PCR amplification • Exponential phase – The signal is in direct proportion to the increase of PCR product – A plot of DNA concentration vs cycle number on a log scale should approximate a straight line

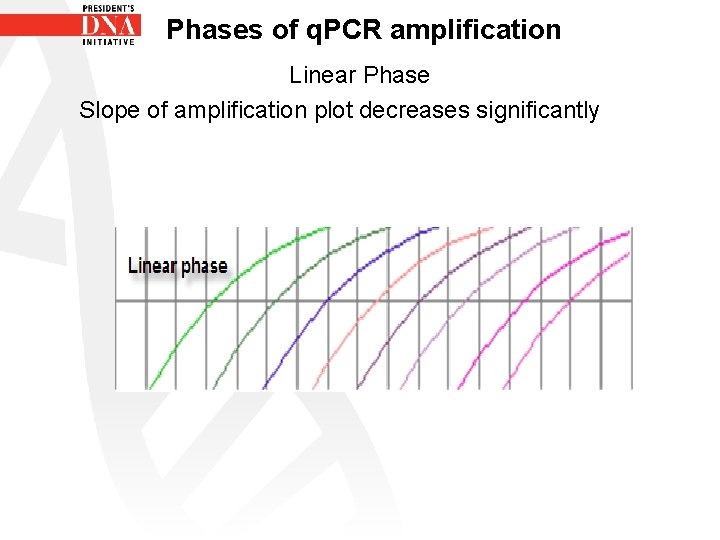
Phases of q. PCR amplification Linear phase – One or more components of PCR have decreased below a critical concentration therefore amplification begins to decrease – Phase is called linear because amplification approximates an arithmetic progression rather than a geometric increase – Amplification efficiency is continually decreasing during linear phase

Phases of q. PCR amplification Linear Phase Slope of amplification plot decreases significantly

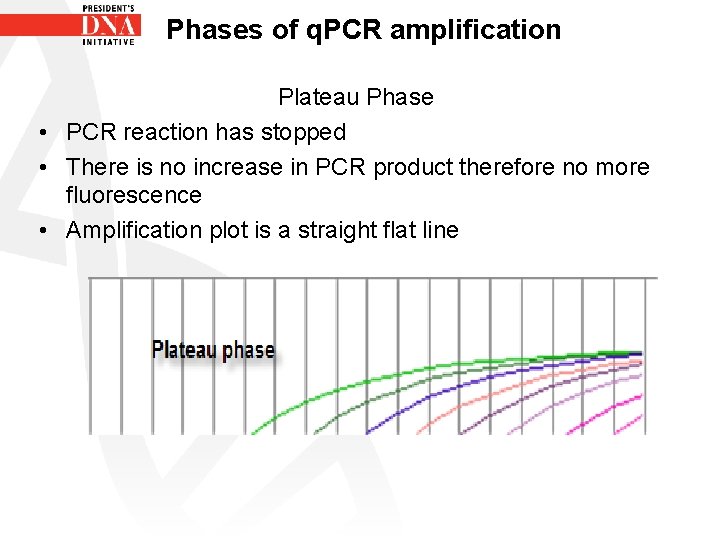
Phases of q. PCR amplification Plateau Phase • PCR reaction has stopped • There is no increase in PCR product therefore no more fluorescence • Amplification plot is a straight flat line

q. PCR Chemistry • Overview – Two 5’ nuclease assays – One for target of Total Human DNA or Human male DNA – One for target of Internal PCR Control (IPC)

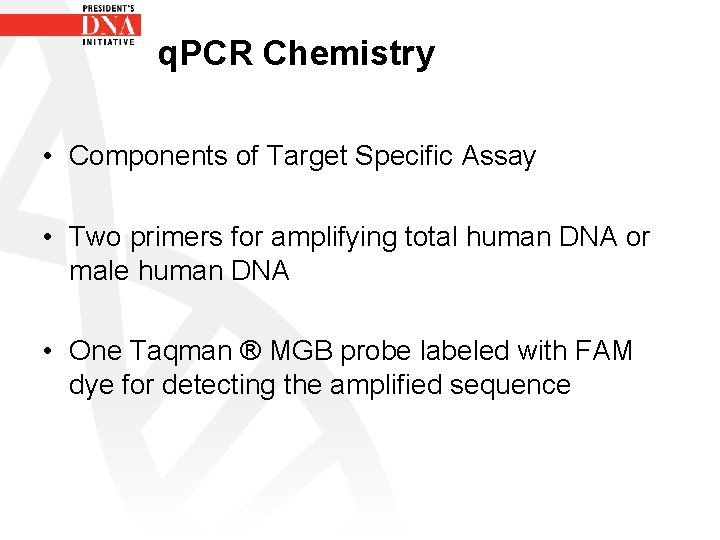
q. PCR Chemistry • Components of Target Specific Assay • Two primers for amplifying total human DNA or male human DNA • One Taqman ® MGB probe labeled with FAM dye for detecting the amplified sequence

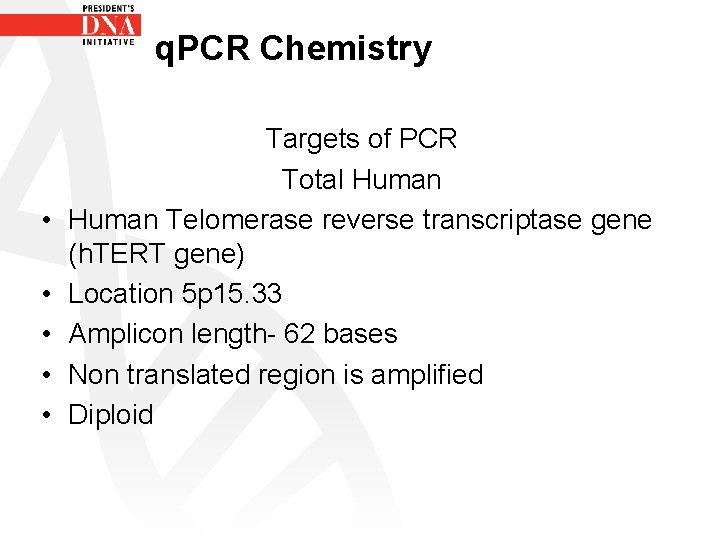
q. PCR Chemistry • • • Targets of PCR Total Human Telomerase reverse transcriptase gene (h. TERT gene) Location 5 p 15. 33 Amplicon length- 62 bases Non translated region is amplified Diploid

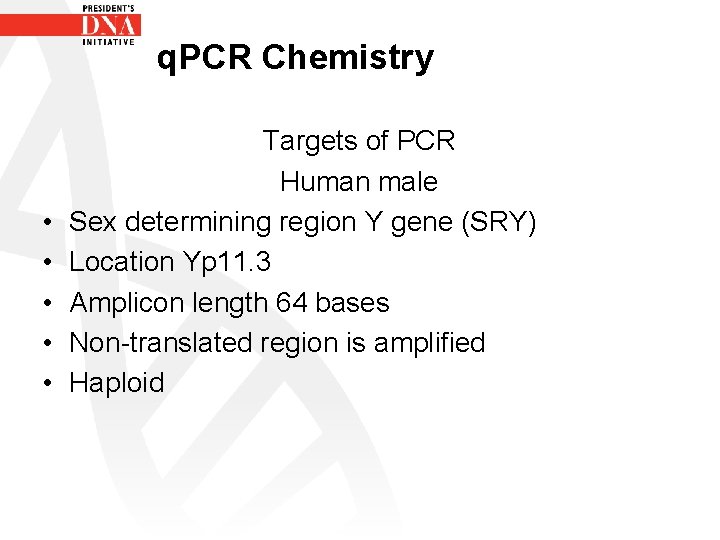
q. PCR Chemistry • • • Targets of PCR Human male Sex determining region Y gene (SRY) Location Yp 11. 3 Amplicon length 64 bases Non-translated region is amplified Haploid

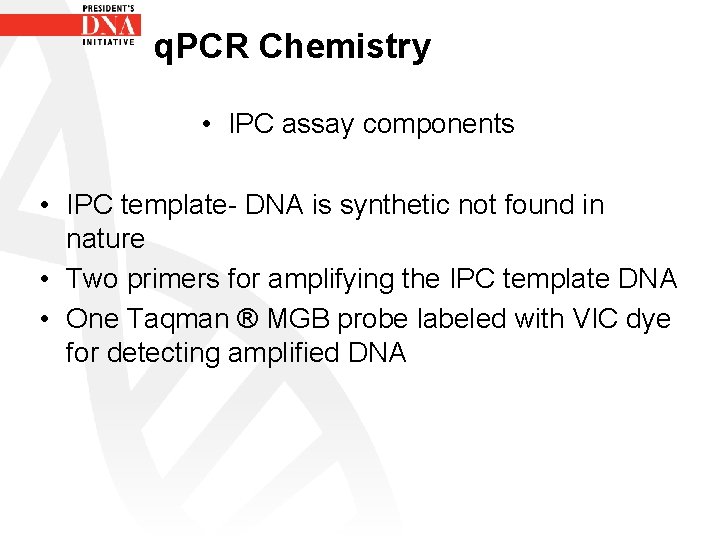
q. PCR Chemistry • IPC assay components • IPC template- DNA is synthetic not found in nature • Two primers for amplifying the IPC template DNA • One Taqman ® MGB probe labeled with VIC dye for detecting amplified DNA

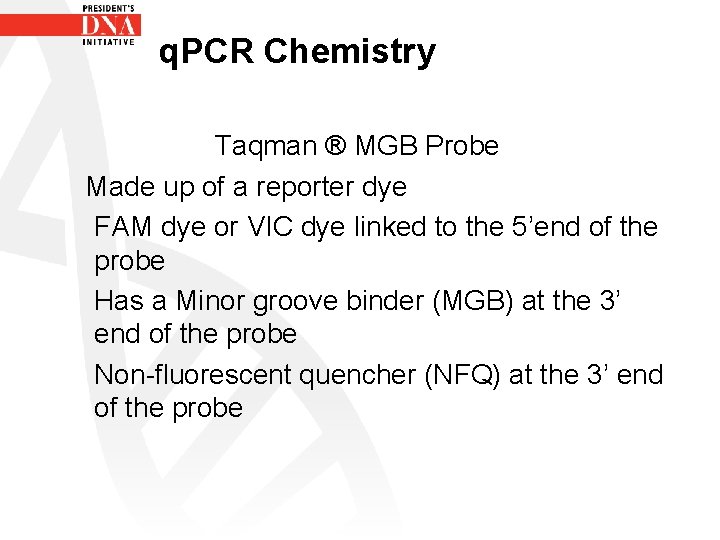
q. PCR Chemistry Taqman ® MGB Probe Made up of a reporter dye FAM dye or VIC dye linked to the 5’end of the probe Has a Minor groove binder (MGB) at the 3’ end of the probe Non-fluorescent quencher (NFQ) at the 3’ end of the probe

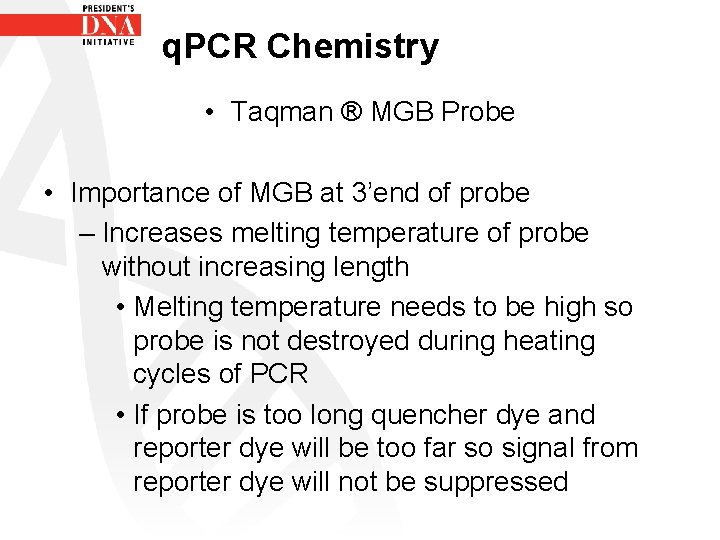
q. PCR Chemistry • Taqman ® MGB Probe • Importance of MGB at 3’end of probe – Increases melting temperature of probe without increasing length • Melting temperature needs to be high so probe is not destroyed during heating cycles of PCR • If probe is too long quencher dye and reporter dye will be too far so signal from reporter dye will not be suppressed

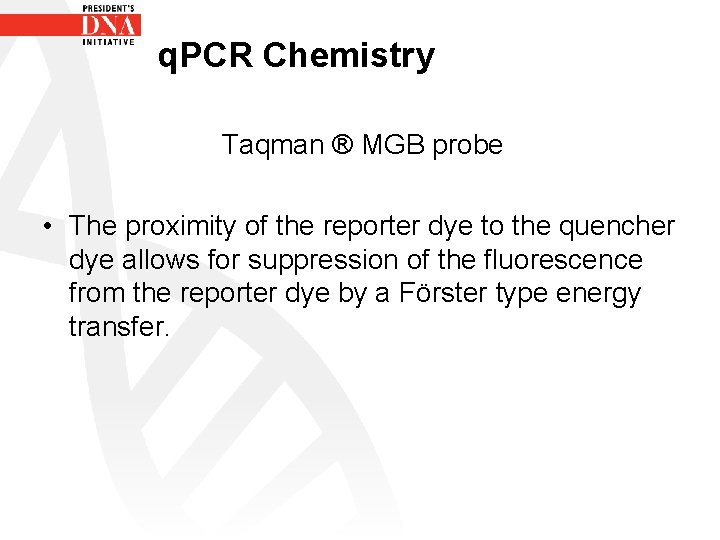
q. PCR Chemistry Taqman ® MGB probe • The proximity of the reporter dye to the quencher dye allows for suppression of the fluorescence from the reporter dye by a Förster type energy transfer.

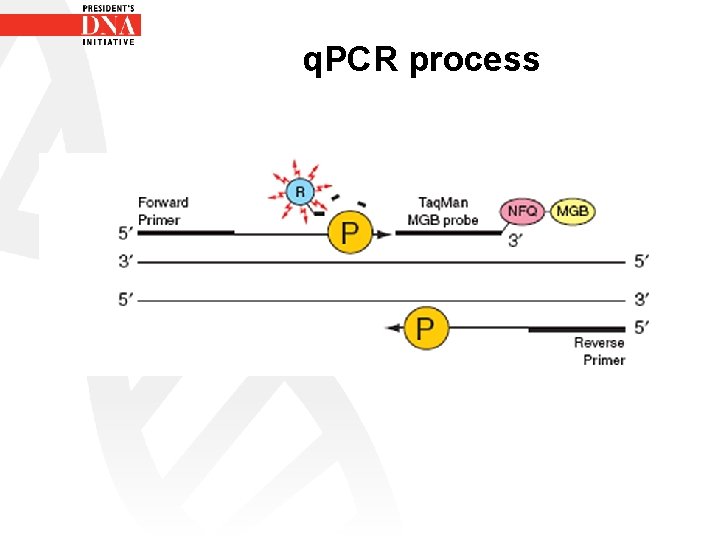
q. PCR process • 5’ nuclease assay process (Nuclease activity is on the 5’ end of the probe) – 1 - Occurs during PCR amplification – 2 - Occurs in every cycle – 3 - Does not interfere with exponential accumulation of product

q. PCR process Components of q. PCR

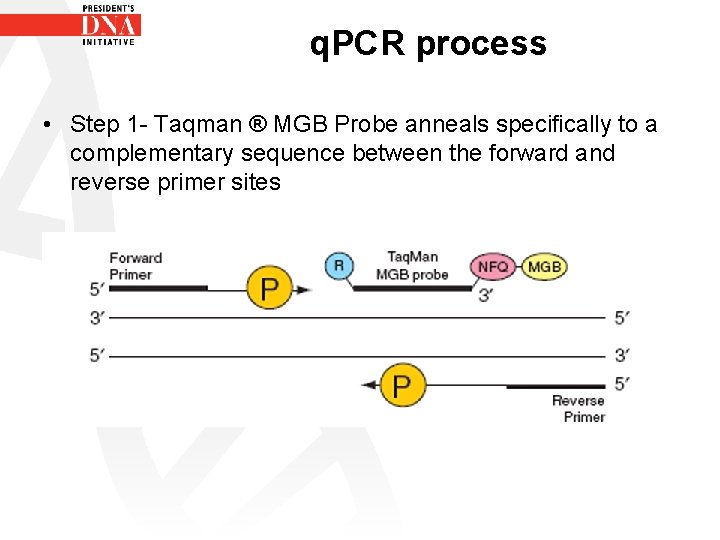
q. PCR process • Step 1 - Taqman ® MGB Probe anneals specifically to a complementary sequence between the forward and reverse primer sites

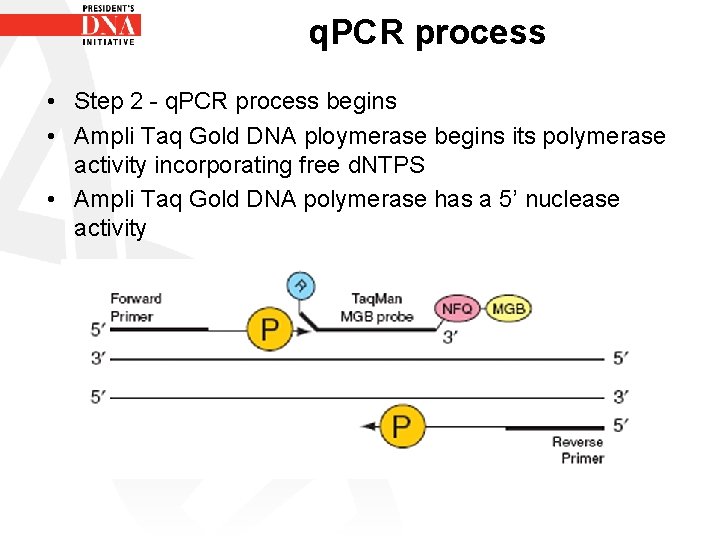
q. PCR process • Step 2 - q. PCR process begins • Ampli Taq Gold DNA ploymerase begins its polymerase activity incorporating free d. NTPS • Ampli Taq Gold DNA polymerase has a 5’ nuclease activity

q. PCR process As Ampli Taq Gold DNA polymerase progresses on the strand of DNA it cleaves off the reporter dye on the 5’ end of probe. As the reporter dye is cleaved it is no longer close enough to the quencher dye for suppression of fluorescence to occur. As a result fluorescence from reporter dye can be detected

q. PCR process

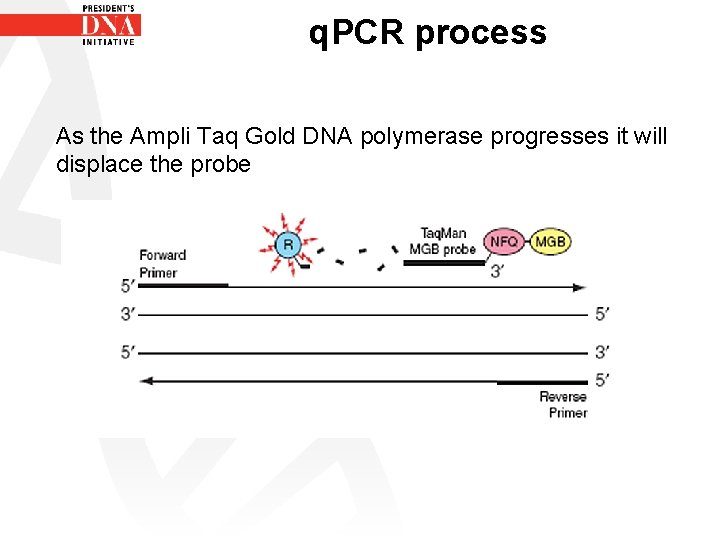
q. PCR process As the Ampli Taq Gold DNA polymerase progresses it will displace the probe

q. PCR process • IPC undergoes same process as template DNA • Because it is a synthetic sequence and labeled with a VIC dye its fluorescence is detected separately

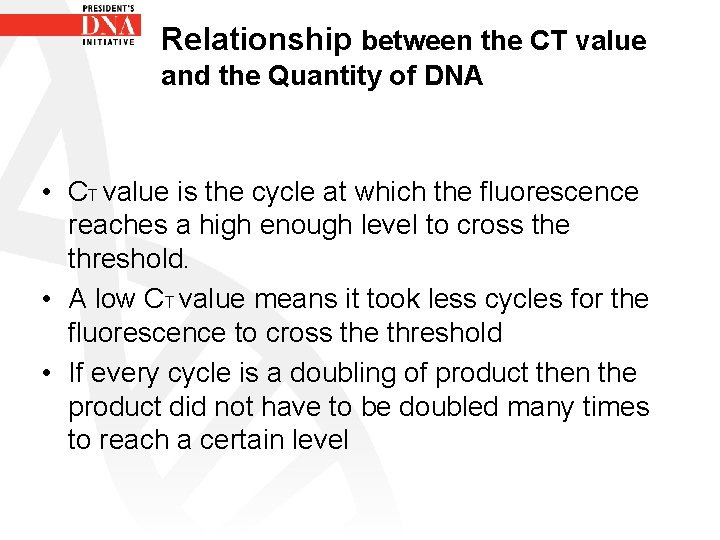
Relationship between the CT value and the Quantity of DNA • CT value is the cycle at which the fluorescence reaches a high enough level to cross the threshold. • A low CT value means it took less cycles for the fluorescence to cross the threshold • If every cycle is a doubling of product then the product did not have to be doubled many times to reach a certain level

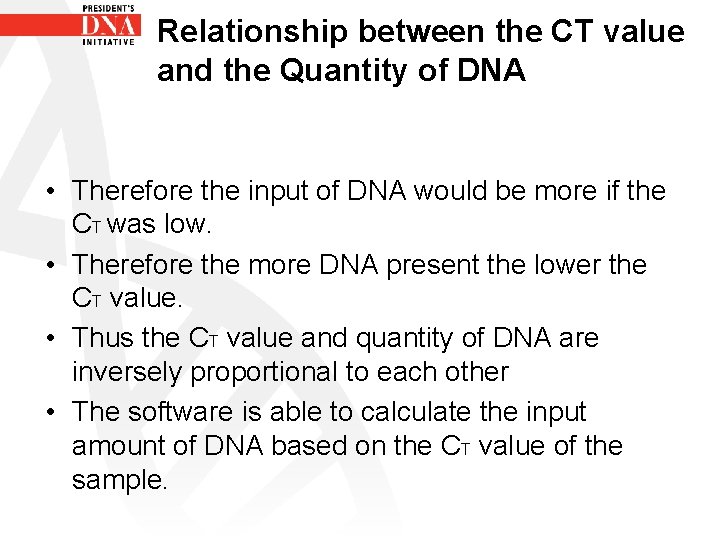
Relationship between the CT value and the Quantity of DNA • Therefore the input of DNA would be more if the CT was low. • Therefore the more DNA present the lower the CT value. • Thus the CT value and quantity of DNA are inversely proportional to each other • The software is able to calculate the input amount of DNA based on the CT value of the sample.

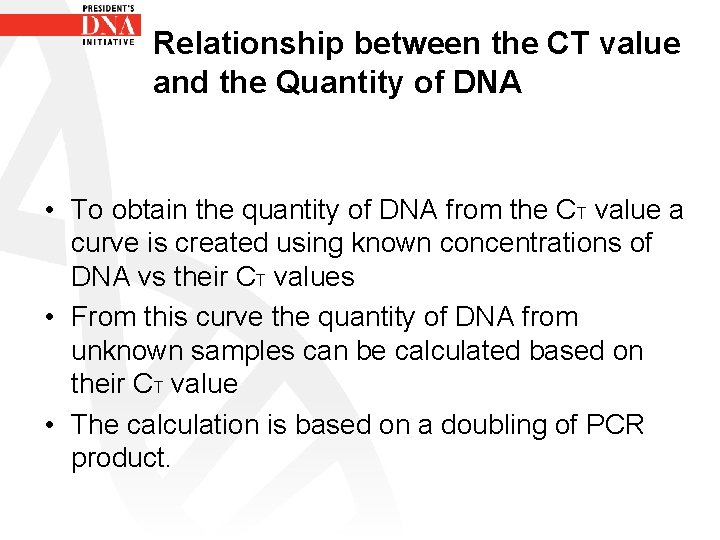
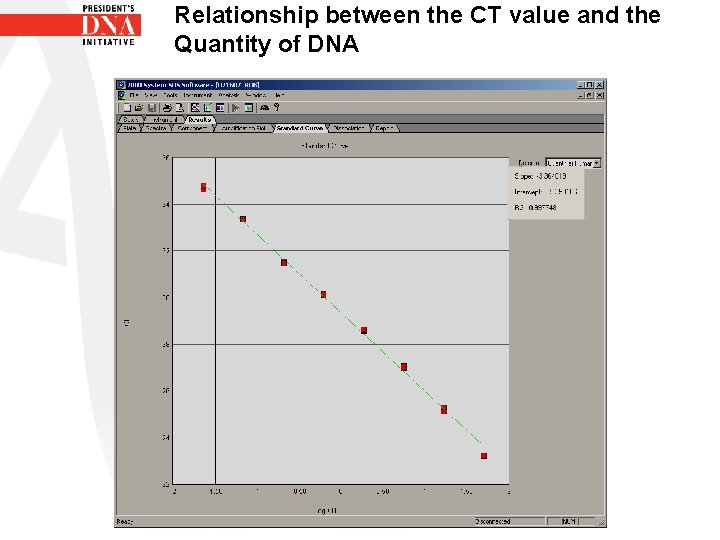
Relationship between the CT value and the Quantity of DNA • To obtain the quantity of DNA from the CT value a curve is created using known concentrations of DNA vs their CT values • From this curve the quantity of DNA from unknown samples can be calculated based on their CT value • The calculation is based on a doubling of PCR product.

Relationship between the CT value and the Quantity of DNA • Therefore the portion of the amplification plot looked at is when the PCR reaction is occurring at 100% efficiency • This occurs during the exponential phase • The standard curve is a graph of the CT of the standards vs the Log of the Concentration of the standards

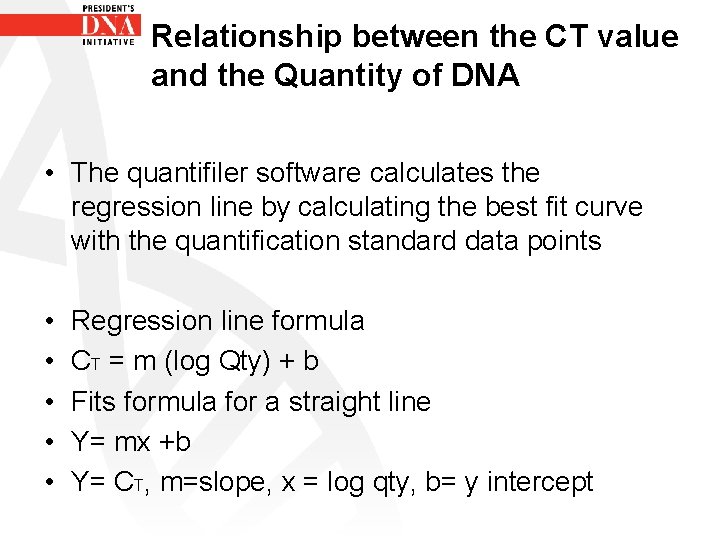
Relationship between the CT value and the Quantity of DNA • The quantifiler software calculates the regression line by calculating the best fit curve with the quantification standard data points • • • Regression line formula CT = m (log Qty) + b Fits formula for a straight line Y= mx +b Y= CT, m=slope, x = log qty, b= y intercept

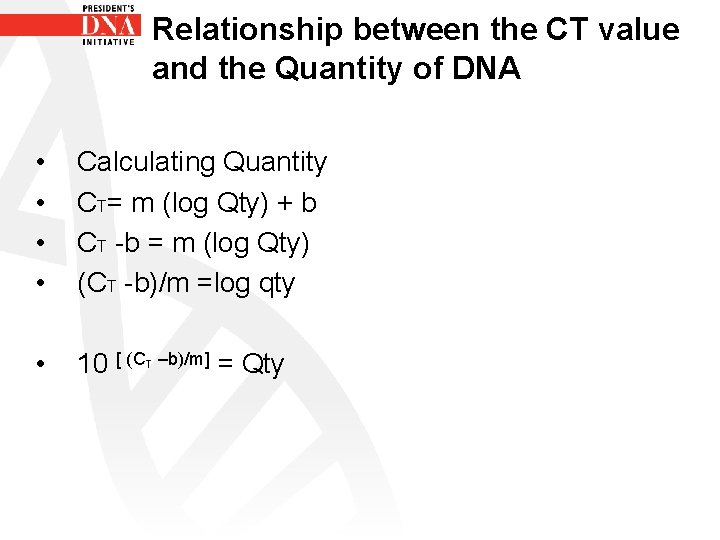
Relationship between the CT value and the Quantity of DNA • • Calculating Quantity CT= m (log Qty) + b CT -b = m (log Qty) (CT -b)/m =log qty • 10 [ (C T –b)/m] = Qty

Relationship between the CT value and the Quantity of DNA • Qty= starting quantity of DNA • R² value- Measure of the closeness of the fit between the standard curve regression line and the individual Ct data points of the standards. A value of 1. 00 means a perfect fit. R² value > 0. 99 represents a close fit. • Slope indicates the PCR amplification efficiency for the assay. • A slope of -3. 3 indicates 100% amplification efficiency. • The Standard curve shows all this information and it is used to assess if the standards for the plate were set up correctly.

Relationship between the CT value and the Quantity of DNA
![Quantitative PCR Reaction efficiency 10 1m 1 Reaction efficiency of 100 Quantitative PCR • • Reaction efficiency = [10 (-1/m) ]-1 Reaction efficiency of 100%](https://slidetodoc.com/presentation_image/4a426d53c89863c25d974e9c7fcb00d5/image-44.jpg)
Quantitative PCR • • Reaction efficiency = [10 (-1/m) ]-1 Reaction efficiency of 100% = 1 1= [10 (-1/m) ]-1 Solving for m (slope) 2 = 10 (-1/m) Log 2 = -1/m -1/log 2 = m -3. 3= m • Y intercept indicates expected CT value for a sample with qty = 1 ng/µl