Quantitative estimation of DNA and RNA Estimation of

















- Slides: 33

Quantitative estimation of DNA and RNA Estimation of nucleotides is the very important step after sample isolation to find out the amount of the nucleotide present and to check for the suitability of the sample for the further analysis. Related Loss: DNA properties > Prior Viewing – IDD-1. Extraction of bacterial protein, IDD-6. Extraction of serum protein > Future Viewing – IDD-17. SDS-PAGE, IDD-33. Western blot assay Course Name: Quantitative estimation of DNA and RNA test Level(UG/PG): UG Author(s): Dinesh Raghu, Vinayak Pachapur Mentor: Dr. Sanjeeva Srivastava *The contents in this ppt are licensed under Creative Commons Attribution-Non. Commercial-Share. Alike 2. 5 India license

1 2 3 4 5 Learning objectives After interacting with this learning object, the learner will be able to: 1. Describe the presence of DNA/RNA in the sample 2. Define the mechanism of detection 3. Operate the steps used in colorimetry 4. Infer the law governing the colorimetric analysis 5. Assess the troubleshooting steps involved in the experiments.

1 2 Definitions and Keywords 1. DNA: Deoxyribonucleic acid, contains the genetic information used in the functioning and development living organisms 2. RNA: are ribonucleic acid, chemical nature is very much similar to DNA, play an active role in cell signaling, carry out biological reactions and even control expression gene. 3. Ethidium bromide: a fluorescent tag binds reversible to DNA molecule which fluoresce with orange color when exposed to UV. 3 4 5

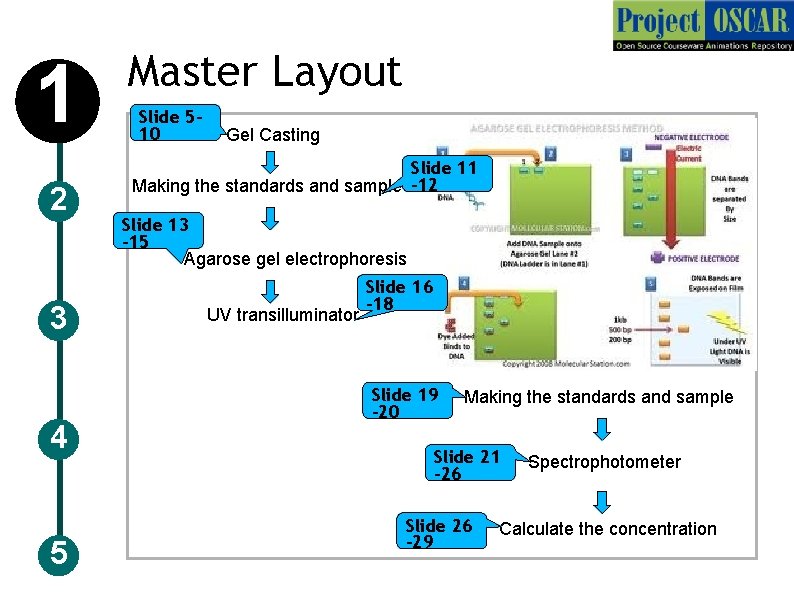
1 2 3 4 5 Master Layout Slide 510 Gel Casting Slide 11 Making the standards and sample -12 Slide 13 -15 Agarose gel electrophoresis UV transilluminator Slide 16 -18 Slide 19 -20 Making the standards and sample Slide 21 -26 Slide 26 -29 Spectrophotometer Calculate the concentration

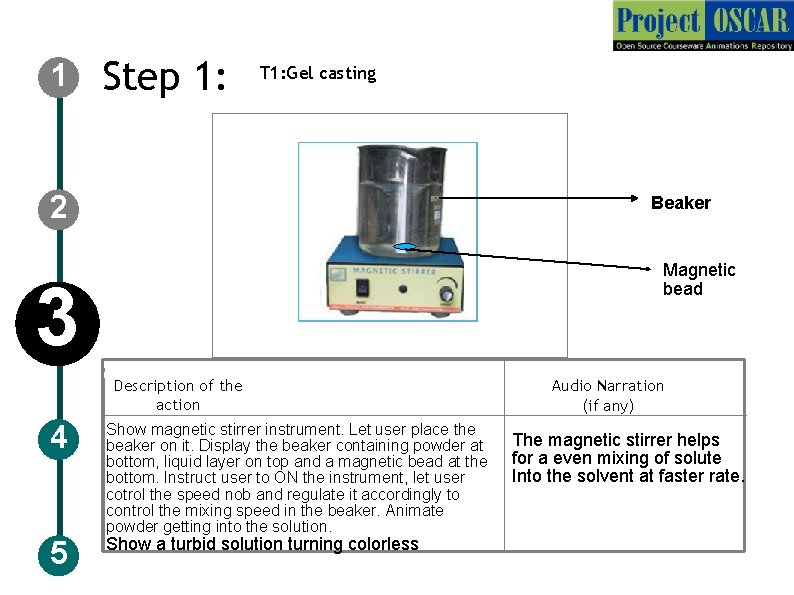
1 Step 1: T 1: Gel casting 2 Beaker Magnetic bead 3 Description of the action 4 5 Show magnetic stirrer instrument. Let user place the beaker on it. Display the beaker containing powder at bottom, liquid layer on top and a magnetic bead at the bottom. Instruct user to ON the instrument, let user cotrol the speed nob and regulate it accordingly to control the mixing speed in the beaker. Animate powder getting into the solution. Show a turbid solution turning colorless Audio Narration (if any) The magnetic stirrer helps for a even mixing of solute Into the solvent at faster rate.

1 Step 1: T 1: Gel casting 2 3 4 5 Measuring balance Description of the action Audio Narration (if any) Show a measuring balance, with display, ON, OFF and TARE/0 buttons on it. let When measuing user ON it, display reading as 0. 000 g, let with paper, the user picks up the paper from the rack, weight of the makes 1/10 of folding on the sides and paper need to be places it on the balance. Now the display tared from actual reading changes to 0. 003 g. Instruct user reading. to TARE the reading. And animate to click the tare button. Once user clicks it, reading must show ” 0”

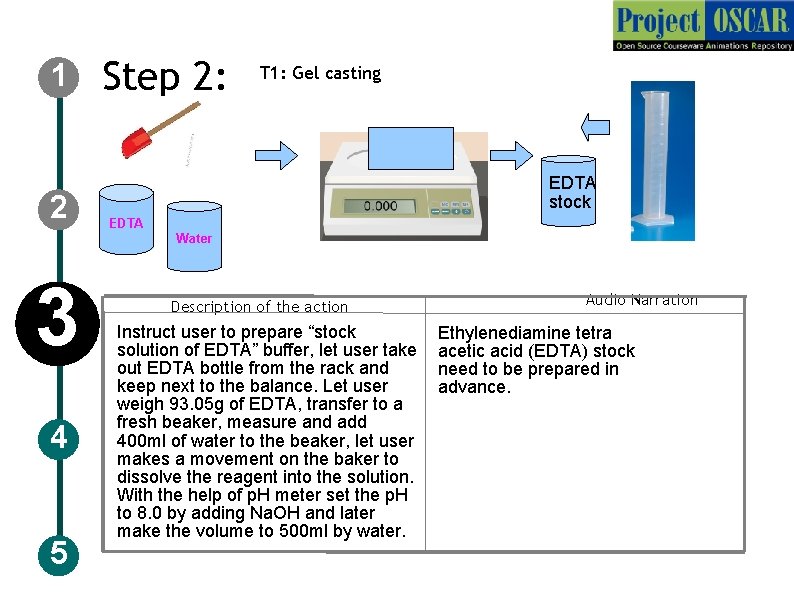
1 2 Step 2: T 1: Gel casting EDTA stock EDTA Water 3 4 5 Description of the action Instruct user to prepare “stock solution of EDTA” buffer, let user take out EDTA bottle from the rack and keep next to the balance. Let user weigh 93. 05 g of EDTA, transfer to a fresh beaker, measure and add 400 ml of water to the beaker, let user makes a movement on the baker to dissolve the reagent into the solution. With the help of p. H meter set the p. H to 8. 0 by adding Na. OH and later make the volume to 500 ml by water. Audio Narration Ethylenediamine tetra acetic acid (EDTA) stock need to be prepared in advance.

1 2 Step 3: T 1: Gel casting TAE stock TRIS Water 3 4 5 Description of the action Instruct user to prepare “stock solution of TBE (50 X)” buffer, let user take out tris base and glacial acetic acid bottle from the rack and keep next to the balance. Let user weigh 242 g of Tris base, transfer to a fresh beaker, measure and add 750 ml water to the beaker, let user makes a movement on the baker to dissolve the reagent into the solution and mixing done as in slide 5. Instruct user to measure and add 57. 1 ml of glacial acetic acid and 100 ml of EDTA stock solution. Let user make up the volume to 1000 ml by water. Audio Narration The stock solution of TBE(50 X) can be stored at room temperature and p. H adjustment is not required. For running the gel prepare 1 X TBE buffer, which can be used for DNA quantification.

1 2 3 4 5 Step 4: T 1: Gel casting TBE stock Tris Boric acid Description of the action Instruct user to prepare “stock solution of TBE (5 X)” buffer, let user take out tris base and boric acid bottle from the rack and keep next to the balance. Let user weigh 54 g of Tris base, 27. 5 g boric acid, transfer to a fresh beaker, measure and add 900 ml water to the beaker, let user makes a movement on the baker to dissolve the reagent into the solution. Instruct user to measure and add 20 ml of EDTA stock solution. Let user make up the volume to 1000 ml by water. Audio Narration The stock solution of TBE(5 X) can be stored at room temperature and p. H adjustment is not required. For running the gel prepare 10 X TBE buffer, which can be used for RNA quantification.

Step 5: 1 T 1: Gel casting 2 Gel solution Agarose 3 4 5 Description of the action Instruct user to take out agarose bottle from the rack and keep next to the balance. Let user weigh 320 mg of agarose and transfer to a fresh 250 ml conical flask. To the flask let user add 40 ml of TBE buffer. Instruct user to take the conical flask for heating in oven for few seconds to dissolve the agarose by opening the oven and placing the flask and watching on the instrument. To the melted agarose add 0. 5 ug/ml of ethidium bromide. Label it as Gel solution. Buffer Audio Narration Ethidium bromide is a fluorescent dye interacts with base pairs of DNA/RNA and forms a complex. The complex get absorbed and emitted which can be acquired at visible spectrum.

1 Step 6: T 1: Gel casting 2 3 4 5 Description of the action Instruct the user to put the gel solution into the gel casting unit. Place the gel casting unit, let user clean it with tissue, place the comb on top of it, pour the gel solution slowly. Keep the setup for 30 min. Events must happen when the user clicks on the hand. Show a change from liquid to solid state Audio Narration Place the gel casting unit, depending upon the sample user can place the comb for the wells. Pour the gel solution into the unit, avoid air bubbles.

1 Step 7: T 2: Making the standards and sample 2 3 4 5 Description of the action Once the gel is cast, let user place the gel in the running unit, pour the 1 X buffer to the required level. Audio Narration Add the running buffer to the unit with the required concentration.

1 Step 8: T 2: Making the standards and sample 2 3 4 5 Description of the action Instruct the user to prepare sample and standard for the loading. Let user take out the dye, sample and DNA/RNA standards from the freezer, keep it on ice for 5 min. In each well, let user mix 1 ul of loading dye(blue in color) with 2 -3 ul of sample/standard. Animate the step, for user taking a pipette setting it to 1 ul, taking out dye, pipette it on parafilm. Now similarly take out sample/standard with pipette, mix it with the dye solution on the paraffin with pipette, after mixing load in the required well and let user make a note of it. Audio Narration The dye and the sample/standard need to be mixed at proper ratio before loading into the gel. The mixing step can be done on parafilm or on eppendrof tube cap. Try to load standards in the first well if possible followed by sample.

1 Step 9: T 3: Agarose gel electrophoresis 2 3 4 5 Description of the action Let user connect the run unit to power supply, let user make proper connection, check for the buffer level, anode and cathode wiring. Make the voltage set for 70 V and click “ON” button. Animate small bubbles coming from the electrode and movement of the blue bands as time passes. Audio Narration Set the required voltage, have a regular check for the buffer level.

1 Step 11: T 3: Agarose gel electrophoresis 2 3 4 5 Description of the action Animate the movement of bands to the 1/3 of the distance of the gel. Instruct user to stop the unit, with help of gloves let user open the lid of the running unit, pick up the gel and take it for UV check. Audio Narration After bands have been resolved in the gel, the gel can be taken for UV visualization.

1 Step 12: T 4: UV transilluminator 2 3 4 5 Gel on UV platform UV instrument Description of the action Show the UV instrument connected to monitor. Let user place open the UV platform drawer, place the gel on the UV platform, close it, let user On the UV light. click on the UV software popup a window, with File, view, edit, save options. Let user click on view>pre-scan. Animate a small window with image appearing row by row to show the scanned image. Animate user to look at the real image, with bands glowing like in gel view under UV. Provide a option for save image, when the scan is completed. Let user name the file accordingly. Scanned image gel view under UV Audio Narration Scan the image at required parameters settings. Let user makes a note of the bands and the readings by saving the file.

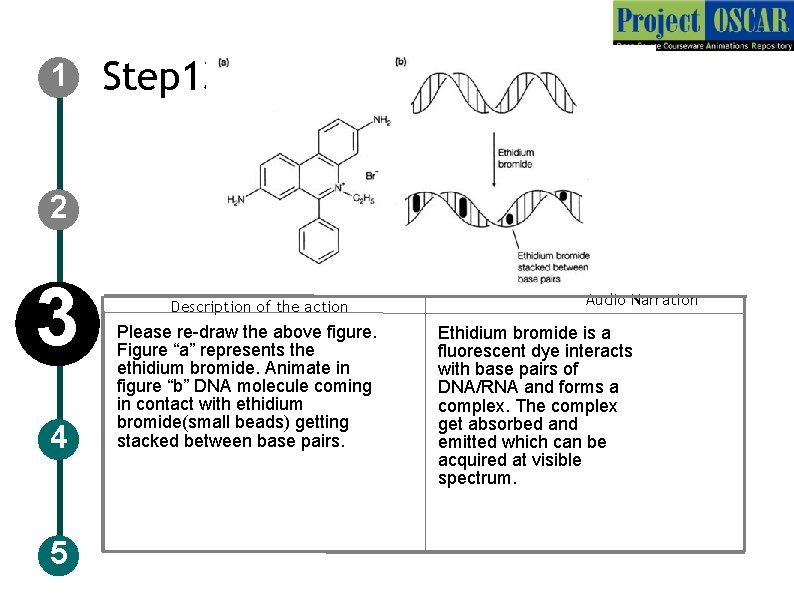
1 Step 13: T 4: UV transilluminator 2 3 4 5 Description of the action Please re-draw the above figure. Figure “a” represents the ethidium bromide. Animate in figure “b” DNA molecule coming in contact with ethidium bromide(small beads) getting stacked between base pairs. Audio Narration Ethidium bromide is a fluorescent dye interacts with base pairs of DNA/RNA and forms a complex. The complex get absorbed and emitted which can be acquired at visible spectrum.

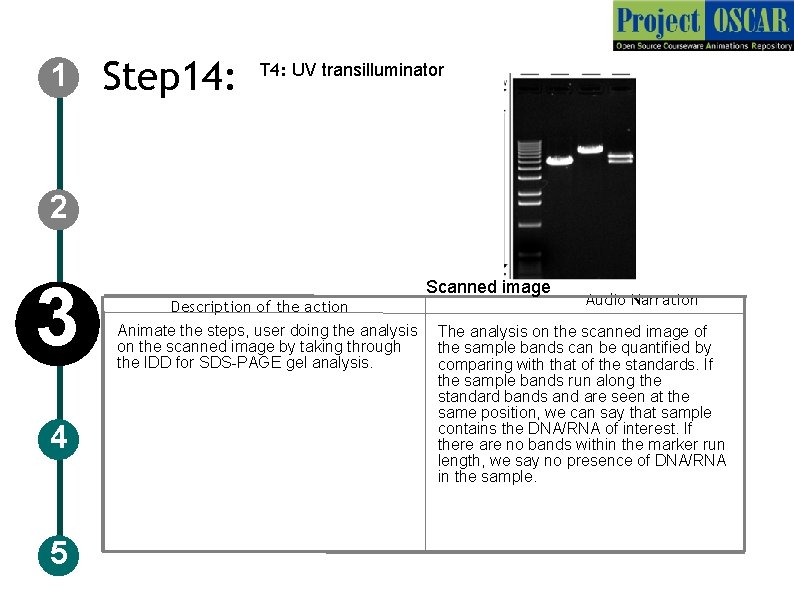
1 Step 14: T 4: UV transilluminator 2 3 4 5 Description of the action Animate the steps, user doing the analysis on the scanned image by taking through the IDD for SDS-PAGE gel analysis. Scanned image Audio Narration The analysis on the scanned image of the sample bands can be quantified by comparing with that of the standards. If the sample bands run along the standard bands and are seen at the same position, we can say that sample contains the DNA/RNA of interest. If there are no bands within the marker run length, we say no presence of DNA/RNA in the sample.

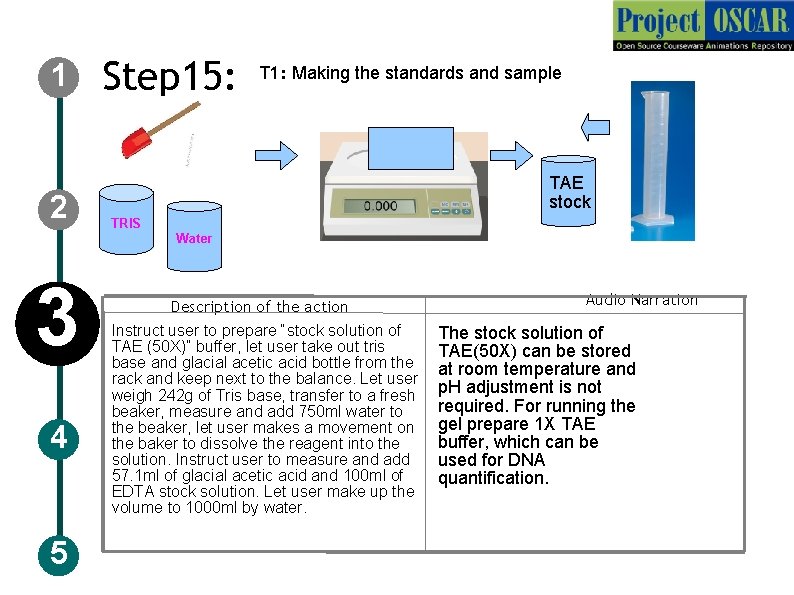
1 2 Step 15: T 1: Making the standards and sample TAE stock TRIS Water 3 4 5 Description of the action Instruct user to prepare “stock solution of TAE (50 X)” buffer, let user take out tris base and glacial acetic acid bottle from the rack and keep next to the balance. Let user weigh 242 g of Tris base, transfer to a fresh beaker, measure and add 750 ml water to the beaker, let user makes a movement on the baker to dissolve the reagent into the solution. Instruct user to measure and add 57. 1 ml of glacial acetic acid and 100 ml of EDTA stock solution. Let user make up the volume to 1000 ml by water. Audio Narration The stock solution of TAE(50 X) can be stored at room temperature and p. H adjustment is not required. For running the gel prepare 1 X TAE buffer, which can be used for DNA quantification.

1 Step 16: T 1: Making the standards and sample 2 3 4 5 Description of the action Instruct user to take out the sample from the -20’C freezer, place them on ice for thawing for 5 min. Let user set the pipeete to 1000 ul to take out TAE buffer and transfer to fresh tube, label it as “BLANK”. Let user set the pipette to 10 ul to take out the sample in fresh tube, now let user set the pipette to 900 ul to add TAE buffer to the tube and label it as “SAMPLE”. Let user close the tube and vortex the tube for proper mixing. Audio Narration Sample for the quantification of DNA must be taken out. After dilution with buffer give a short vortex to mix the solution. Now the solution is ready for the spectrometer reading.

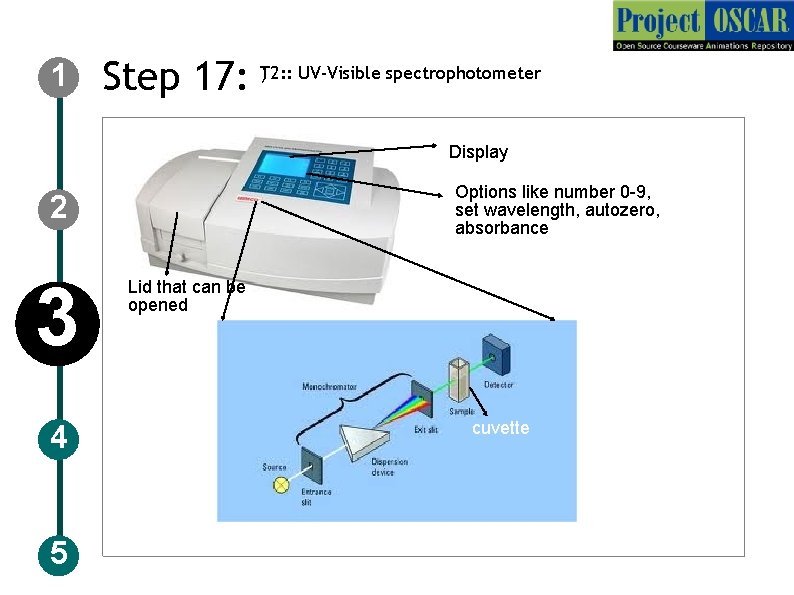
1 ) UV-Visible spectrophotometer Step 17: T 2: : Display Options like number 0 -9, set wavelength, autozero, absorbance 2 3 4 5 Lid that can be opened cuvette

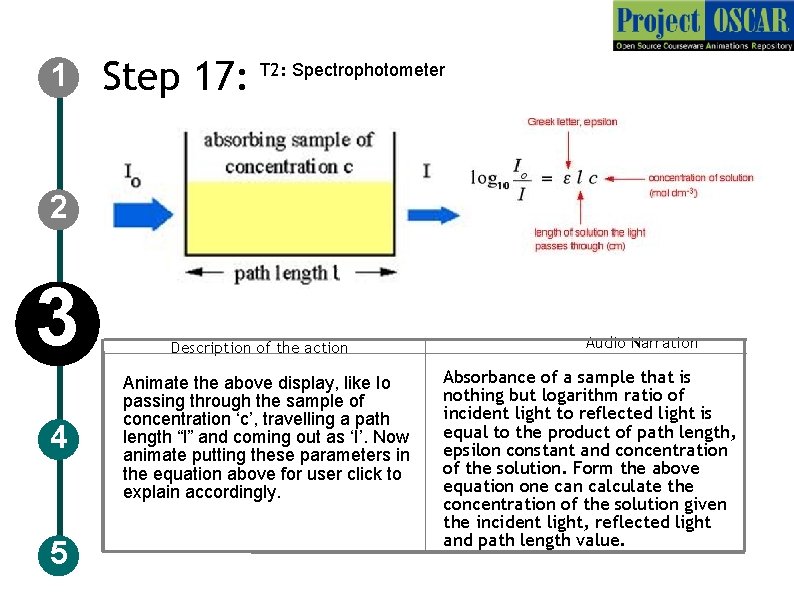
1 2 3 4 5 Step 17: T 2: : UV-Visible spectrophotometer Description of the action Animate the instrument as in figure and redraw the instruments with the specification mentioned in the figure and zoom the instrument and show a schematic as shown in the figure with the labelings but redraw completely Audio Narration UV-Visible spectrophotometer has a monochromator, light source and sample holder and detector, Light from the source are converted to a monochromatic light of particular wavelength and allow it pass through the sample and amount of light that emerges is detected by a detector.

1 Step 17: T 2: Spectrophotometer 2 3 4 5 Description of the action Animate the above display, like Io passing through the sample of concentration ‘c’, travelling a path length “l” and coming out as ‘I’. Now animate putting these parameters in the equation above for user click to explain accordingly. Audio Narration Absorbance of a sample that is nothing but logarithm ratio of incident light to reflected light is equal to the product of path length, epsilon constant and concentration of the solution. Form the above equation one can calculate the concentration of the solution given the incident light, reflected light and path length value.

1 2 3 4 5 Step 18: T 2: Spectrophotometer Description of the action Let the user take the tube 1 and take 2 cuvettes as in figure. Instruct the user to press the open lid show two opening inside it one after the other in longitudinal way. Show like pouring the contents from tube 1 to both the cuvettes , show like taking the tissue and wiping on the sides and placing it in the openings, now animate like closing the lid and press absorbance. (before keeping the cuvette the reading should be 0. 000, once the cuvette is kept and “absorbance is pressed it should be 0. 123) Now instruct the user to press”auto zero” and the reading should be 0. 000 and remove the cuvettes by opening the lid and taking out the cuvette from opening 2 throw the solution out from the cuvette Audio Narration Auto zero and callibrate the instrument using the control solution without hydrogen peroxide

1 Step 22: T 2: Spectrophotometer Description of the action 2 3 4 5 Repeat the same steps like in slide: 25 to set the instrument at 280 nm. This time animate the reading of the sample around 2. 3. let user makes a note of the reading. Also user should take reading at 260 nm. Audio Narration Set the instrument at 280 nm to set it at auto zero with buffer and taking the reading for the sample at 260 nm.

1 Step 24: T 3: Calculate the concentration Description of the action 2 3 4 5 Instruct user to do the ratio calculation of 260/280. animate the ration being displayed on the screen with audio narration. Audio Narration • A ratio value between 1. 8 -2. 0 denotes, presence of nucleic acids. • A ratio value lower than 1. 8 denotes presence of proteins and/or other UV absorbers. • A ratio value higher than 2. 0 indicates that the samples may be contaminated with chloroform or phenol. • In either case (<1. 8 or >2. 0) it is advisable to re-precipitate the DNA.

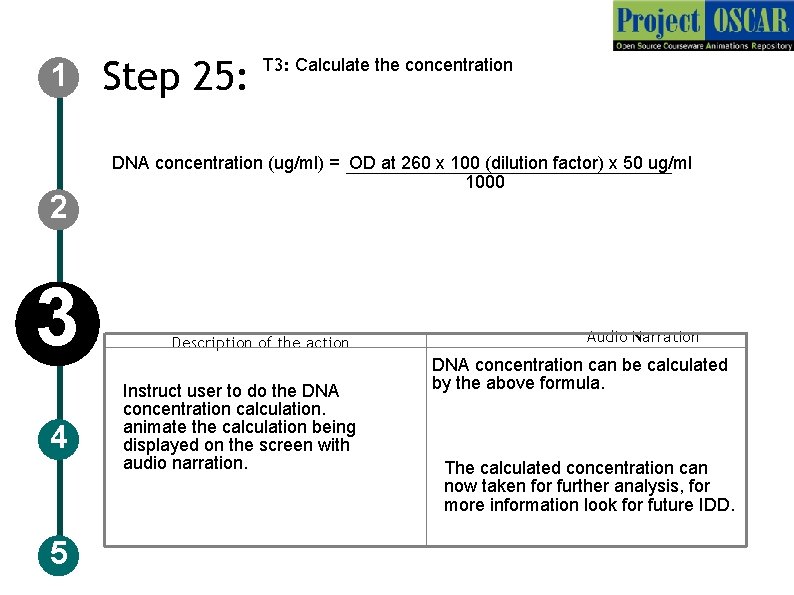
DNA concentration ( g/ml) = OD x 100 (dilution factor) x 50 g/ml 260 1000 1 2 3 4 5 Step 25: T 3: Calculate the concentration DNA concentration (ug/ml) = OD at 260 x 100 (dilution factor) x 50 ug/ml 1000 Description of the action Instruct user to do the DNA concentration calculation. animate the calculation being displayed on the screen with audio narration. Audio Narration DNA concentration can be calculated by the above formula. The calculated concentration can now taken for further analysis, for more information look for future IDD.

Slide 510 Tab 01 Slide 11 -12 Tab 02 Slide 13 -15 Tab 03 Slide 16 -18 Tab 04 Tab 05 Tab 06 Tab 07 Name of the section/stage Interactivity area Animation area In slide-17: provide user a gel image with bands in sample well appearing after the DNA standards bands. Let user interrupt the result? Instruction: user must come out with the solution pointing out presence of proteins and no DNA/RNA in the sample. Must go back to sample extraction step to make the changes. Button 01 Button 02 Button 03 Instructions/ Working area Credits

Slide 19 19 -20 Tab 01 Slide 21 21 -26 Slide 26 -29 Tab 02 Tab 03 Tab 04 Tab 05 Tab 06 Tab 07 Name of the section/stage Interactivity area Animation area Button 01 Button 02 Button 03 Instructions/ Working area Credits

APPENDIX 1 Questionnaire: Question 1 Native DNA, molecular weight a) Greater than (40 kb) b) less than (40 kb) c) Greater than (20 kb) d) Greater than (80 kb) Question 2 the bound dye itself absorbs radiation at a) 366 nm b) 460 nm c) 480 nm d) 302 nm and 366 nm Question 3 The visible spectrum of DNA-Dye complex falls in a) Orange region b) reddish-orange region c) Red region d) Yellow region

APPENDIX 1 Questionnaire: Question 4 In either case 460/480 ratio value (<1. 8 or >2. 0) a) re-precipitate the sample. b) precipitate the sample. c) Mix the sample d) Add buffer Question 5: As the absorbance increases , the intensity of the outgoing light a) b) c) d) Decreases Increases Remains same zero

APPENDIX 2 Links for further reading Reference websites: http: //www. youtube. com/watch? v=6 m. QGNDn. Oy H 8&feature=related Book Hoisington, D. Khairallah, M. and Gonzalez-de-Leon, D. (1994). Laboratory Protocols: CIMMYT Applied Biotechnology Center. Second Edition, Mexico, D. F. : CIMMYT.

APPENDIX 3 Summary The method mostly involves the quantitative and qualitative estimation of DNA/RNA using absorbance ratio method and by agarose electrophoresis. The quantification and estimation of the DNA/RNA can be depend lot on standards and gel concentration used.