Quantitative chemistry The mole amount of chemical substance

Quantitative chemistry
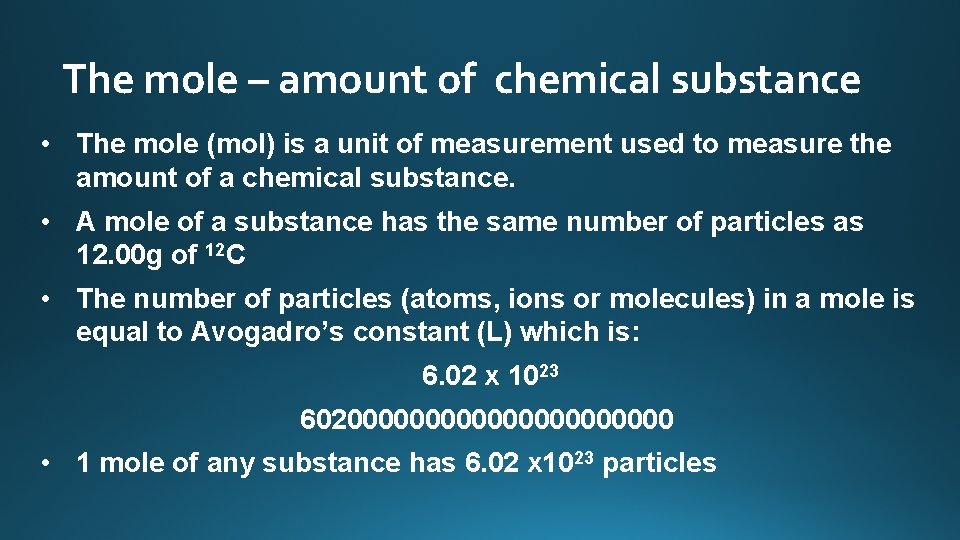
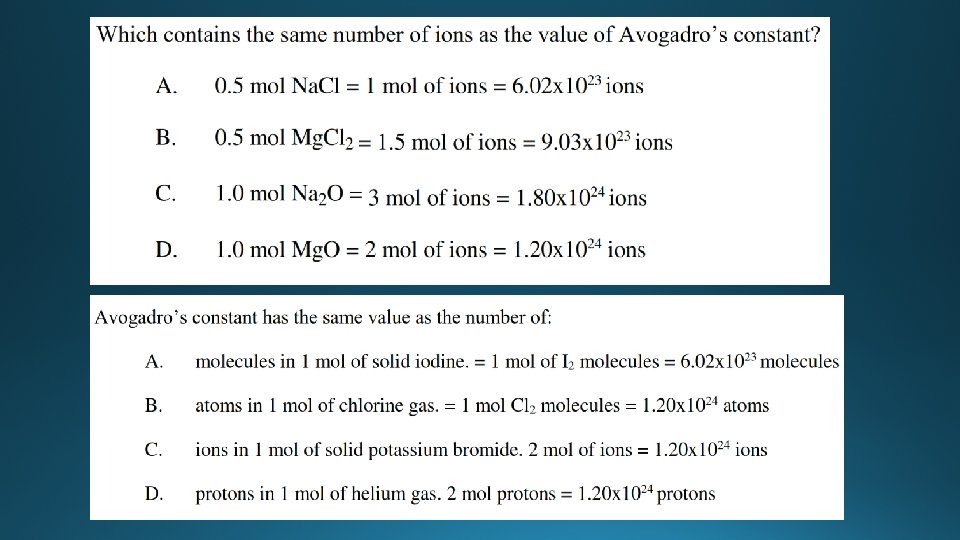
The mole – amount of chemical substance • The mole (mol) is a unit of measurement used to measure the amount of a chemical substance. • A mole of a substance has the same number of particles as 12. 00 g of 12 C • The number of particles (atoms, ions or molecules) in a mole is equal to Avogadro’s constant (L) which is: 6. 02 x 1023 60200000000000 • 1 mole of any substance has 6. 02 x 1023 particles
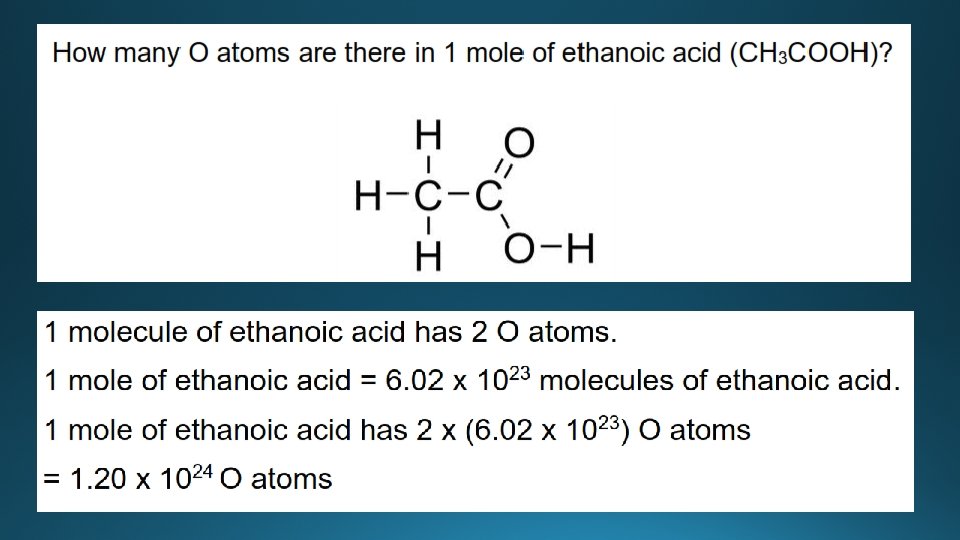
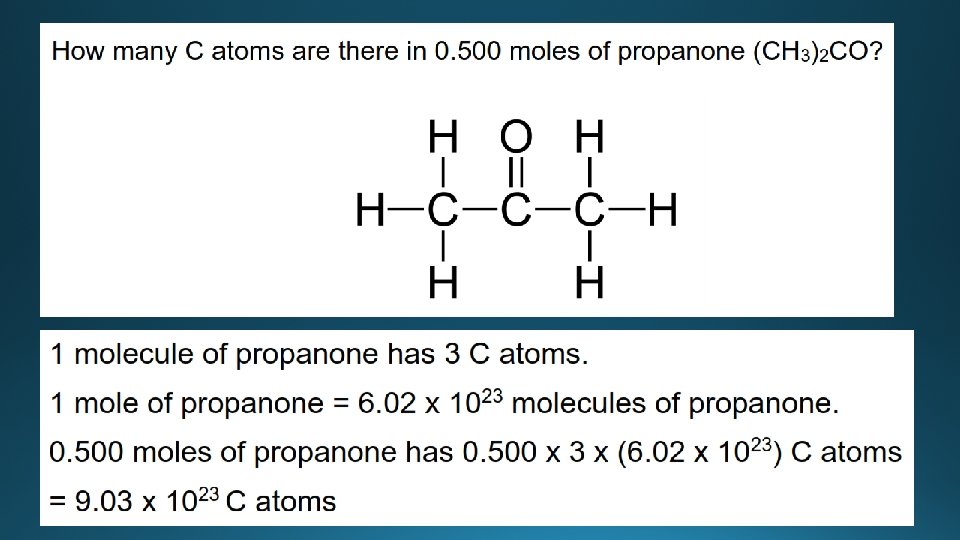

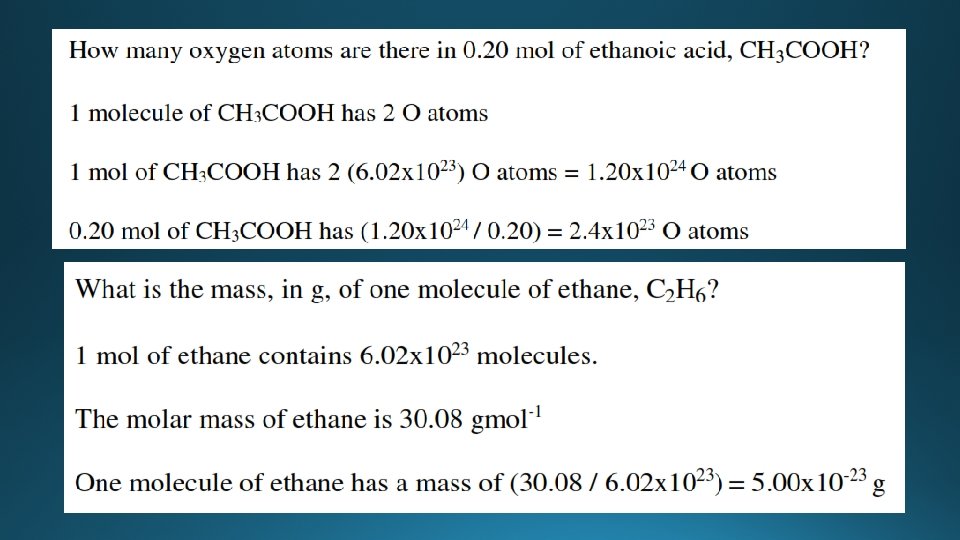
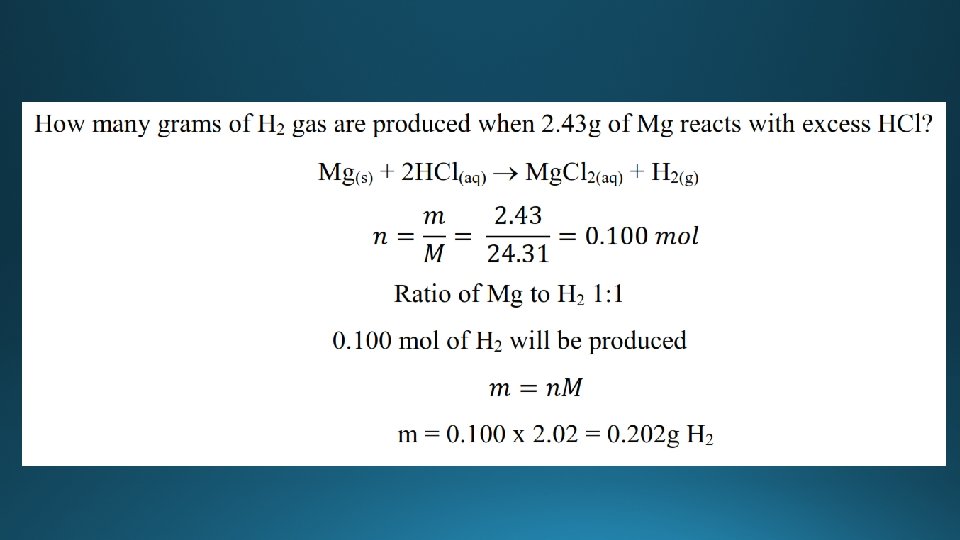
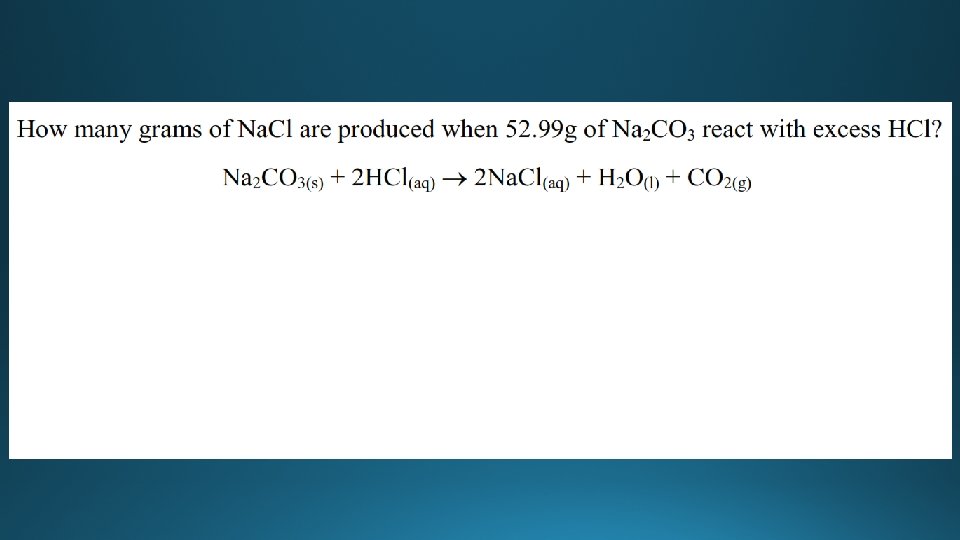
Examples
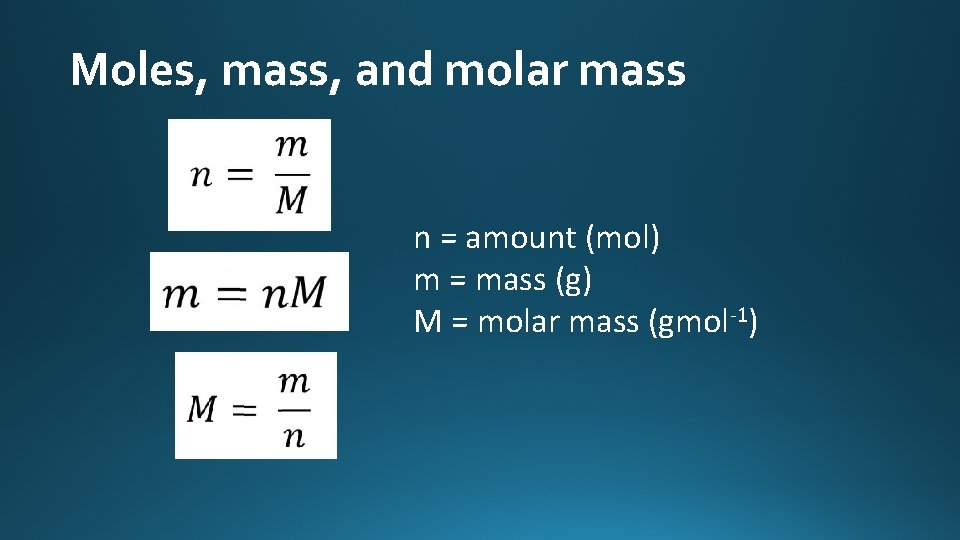
Moles, mass, and molar mass n = amount (mol) m = mass (g) M = molar mass (gmol-1)



• Molar mass is the mass in grams of one mole of substance (gmol-1) • It is calculated by adding up the masses of each atom in a compound. • Example: H 2 O • Hydrogen (H) = 1. 01, oxygen (O) = 16. 00 • M (H 2 O) = (2 x 1. 01) + 16. 00 = 18. 02 gmol-1
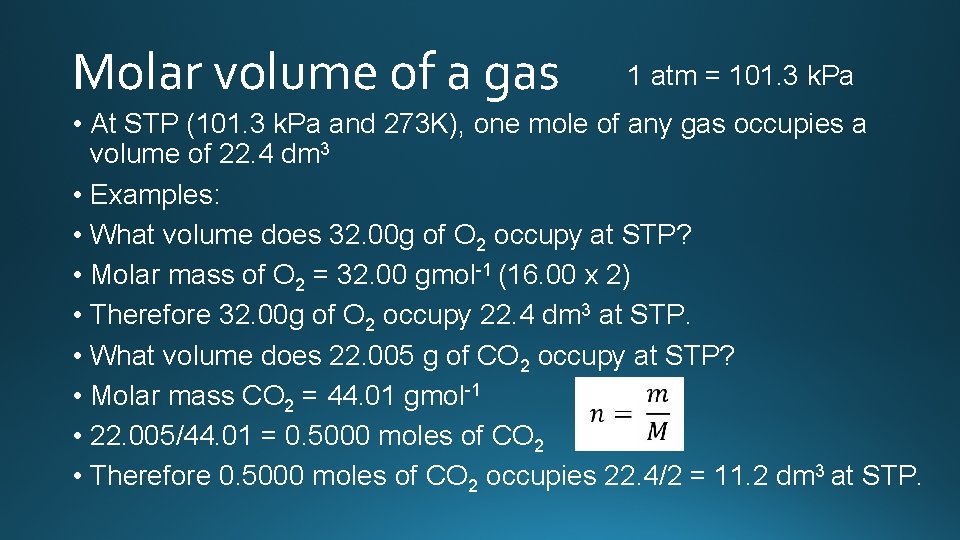
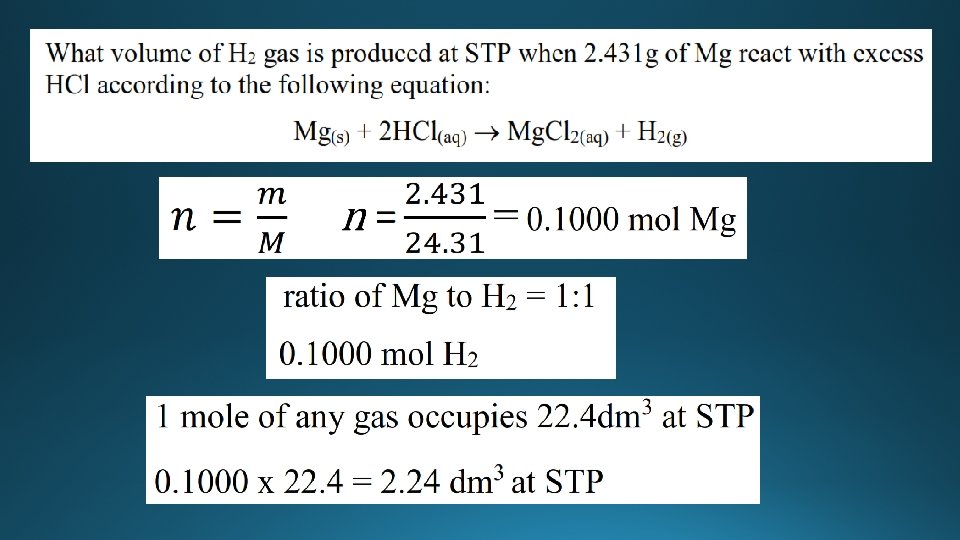
Molar volume of a gas 1 atm = 101. 3 k. Pa • At STP (101. 3 k. Pa and 273 K), one mole of any gas occupies a volume of 22. 4 dm 3 • Examples: • What volume does 32. 00 g of O 2 occupy at STP? • Molar mass of O 2 = 32. 00 gmol-1 (16. 00 x 2) • Therefore 32. 00 g of O 2 occupy 22. 4 dm 3 at STP. • What volume does 22. 005 g of CO 2 occupy at STP? • Molar mass CO 2 = 44. 01 gmol-1 • 22. 005/44. 01 = 0. 5000 moles of CO 2 • Therefore 0. 5000 moles of CO 2 occupies 22. 4/2 = 11. 2 dm 3 at STP.

Relative atomic mass and relative molecular mass • Relative atomic mass (Ar) is defined as the average mass of an atom compared to 1/12 the mass of one atom of carbon-12. • Relative molecular mass (Mr) is defined as the average mass of a molecule compared to 1/12 the mass of one atom of carbon-12. • Both are relative scales and therefore have no units.

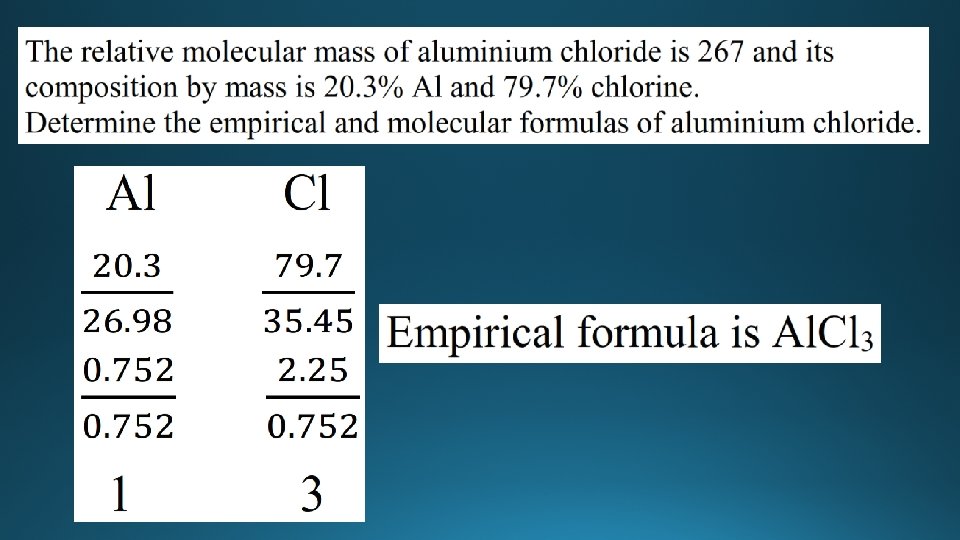
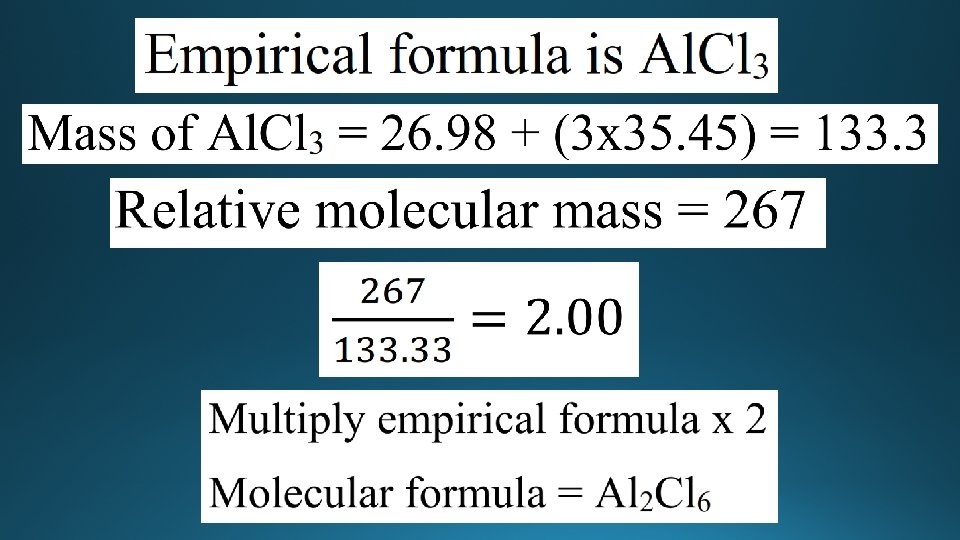
Empirical and molecular formula • Empirical formula is defined as the lowest whole number ratio of atoms in a compound. • Molecular formula is the actual number of atoms in a compound. • Example: • Butane has the molecular formula C 4 H 10 • The empirical formula is C 2 H 5
Molar mass • Molar mass is the mass of one mole of a substance in grams. • The unit is gmol-1 • Examples: • The molar mass of Na. Cl is (22. 99 + 35. 45) = 58. 44 gmol-1 • The molar mass of butane (C 4 H 10) • (4 x 12. 01) + (10 x 1. 01) = 58. 14 gmol-1

Avogadro’s law • Avogadro’s law states that equal volumes of gases at the same temperature and pressure contain the same number of particles. • What volume of CO 2 is produced when 10 dm 3 of CO reacts with 10 dm 3 of O 2? • 2 volumes of CO react with 1 volume of O 2 to make 2 volumes of CO 2 • Ratio of CO to O 2 is 2: 1 – 10 dm 3 of CO reacts with 5 dm 3 of O 2 • There is excess O 2 (CO is limiting reactant) so 10 dm 3 of CO 2 is produced.

Avogadro’s law • What volume of CO 2 is produced when 100 cm 3 of C 2 H 4 is reacted with 400 cm 3 of O 2? What volume of O 2 remains? • 1 volume of C 2 H 4 reacts with 3 volumes of O 2 to produce 2 volumes of CO 2 and H 2 O • Ratio of C 2 H 4 to O 2 is 1: 3 – 100 cm 3 of C 2 H 4 reacts with 300 cm 3 of O 2 • O 2 is excess (C 2 H 4 is limiting reactant) • Ratio of C 2 H 4 to CO 2 is 1: 2, therefore (2 x 100) 200 cm 3 of CO 2 is produced • Volume of O 2 remaining (400 -300) = 100 cm 3

Gas laws • Pressure and volume (Boyle’s law) • Temperature and volume (Charles’ law) • Temperature and pressure (Gay Lussac’s law) • The combined gas law • Ideal gas law

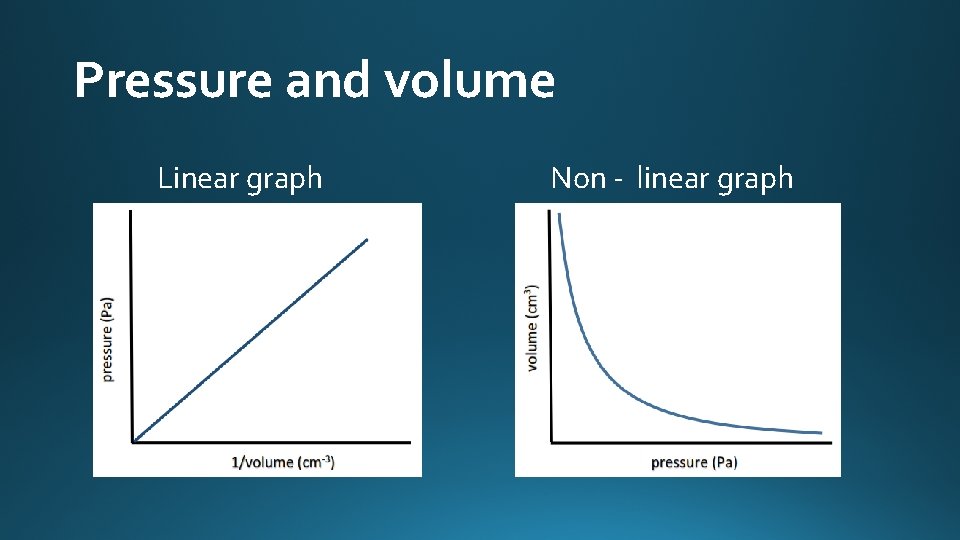
Pressure and volume • Pressure is inversely proportional to volume at constant temperature. • If the volume of a fixed mass of an ideal gas is doubled, the pressure decreases by half. • Example: • What is the new volume of 6 dm 3 of gas if the pressure is halved at constant temperature? • Halve the pressure, the volume is doubled. • New volume = 12 dm 3
Pressure and volume Linear graph Non - linear graph
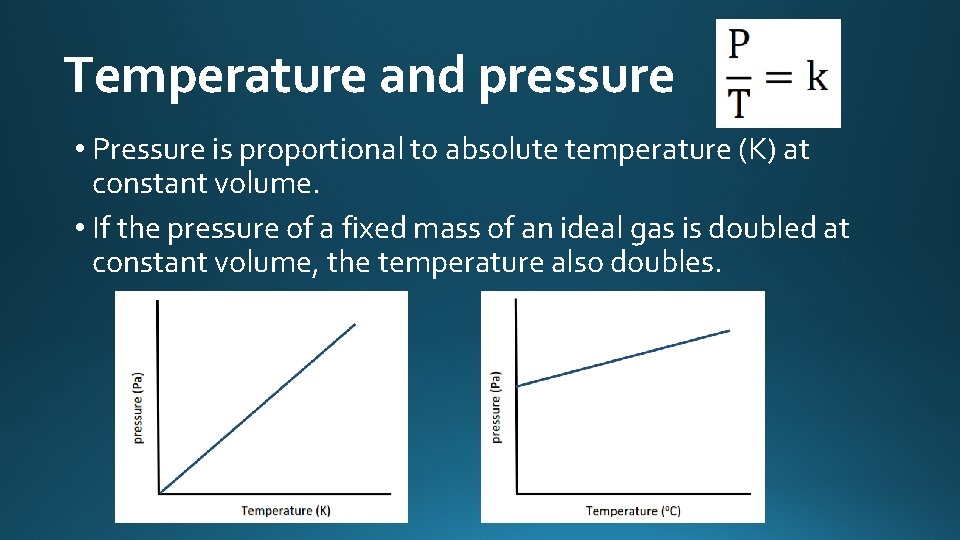
Temperature and pressure • Pressure is proportional to absolute temperature (K) at constant volume. • If the pressure of a fixed mass of an ideal gas is doubled at constant volume, the temperature also doubles.
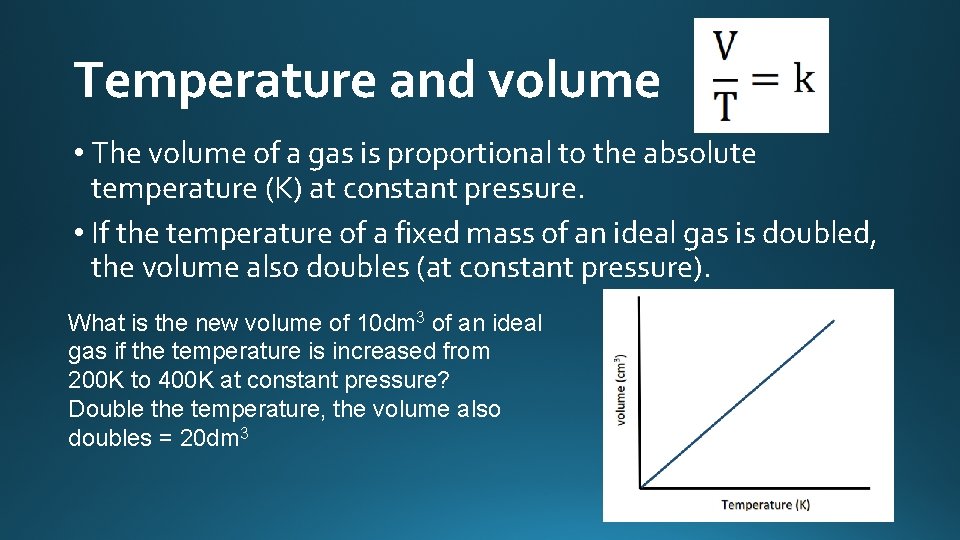
Temperature and volume • The volume of a gas is proportional to the absolute temperature (K) at constant pressure. • If the temperature of a fixed mass of an ideal gas is doubled, the volume also doubles (at constant pressure). What is the new volume of 10 dm 3 of an ideal gas if the temperature is increased from 200 K to 400 K at constant pressure? Double the temperature, the volume also doubles = 20 dm 3

• What happens to a fixed mass of an ideal gas when the pressure and temperature are both doubled? • Pressure is doubled, volume is halved. • Temperature is doubled, volume is doubled. • Therefore the volume does not change. • The volume of an ideal gas at 27. 0 °C is increased from 3. 00 dm 3 to 6. 00 dm 3. At what temperature, in °C, will the gas have the original pressure? • Volume is doubled, pressure is halved • To get back to the original pressure the temperature can be doubled (convert 27 o. C to K = 300 K) • Double 300 K = 600 K, then convert back to o. C (600 -273) = 327 o. C
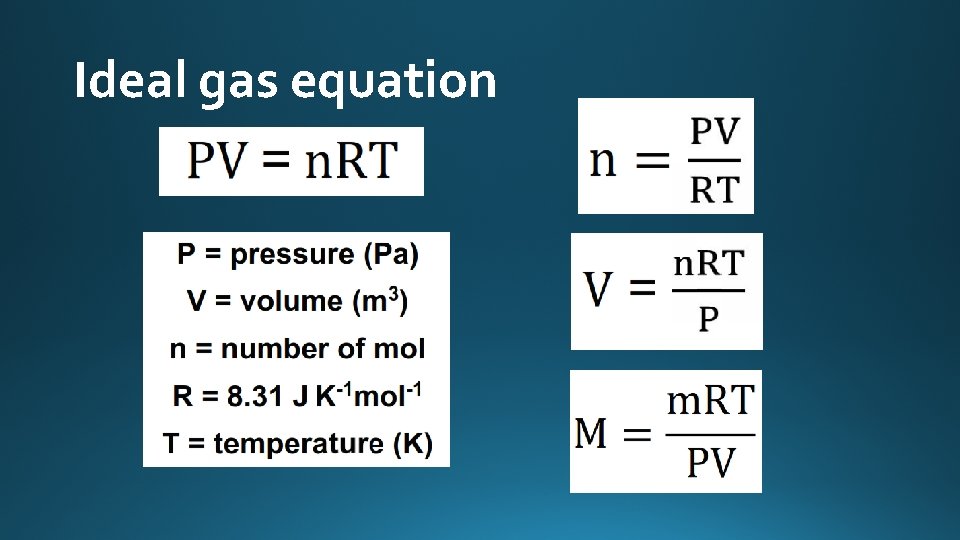
Ideal gas equation

Ideal gas equation

Converting to 3 cm 3 dm to 3 m 3 m divide by 6 1 x 10 divide by 3 1 x 10

Convert the following into m 3 3 78. 4 cm 456 cm 3 3 38. 6 dm 3 198. 78 dm

0 o. C = 273 K Convert the following to K o 5 C 23 o. C o 100 C

1 atm = 5 1. 01 x 10 Convert the following to Pa 10. 0 atm 150. 6 atm 27. 3 atm Pa

Concentration of solutions

Solute, solvent and solution • Solute – the substance that dissolves in the solvent. • Solvent – the liquid that the solute dissolves in. • Solution – solute + solvent together. • When Copper sulfate is dissolved in water, a blue solution is formed. State the solute and the solvent. • Copper sulfate is the solute and water is the solvent.

Calculating concentration moldm-3 number of moles volume of solvent (dm 3) volume must be in dm 3 (divide cm 3 by 1000 to get dm 3)

Example 1 Find the concentration of a solution containing 20. 0 g of sodium hydroxide (Na. OH) in 200. 0 cm 3 of solution.
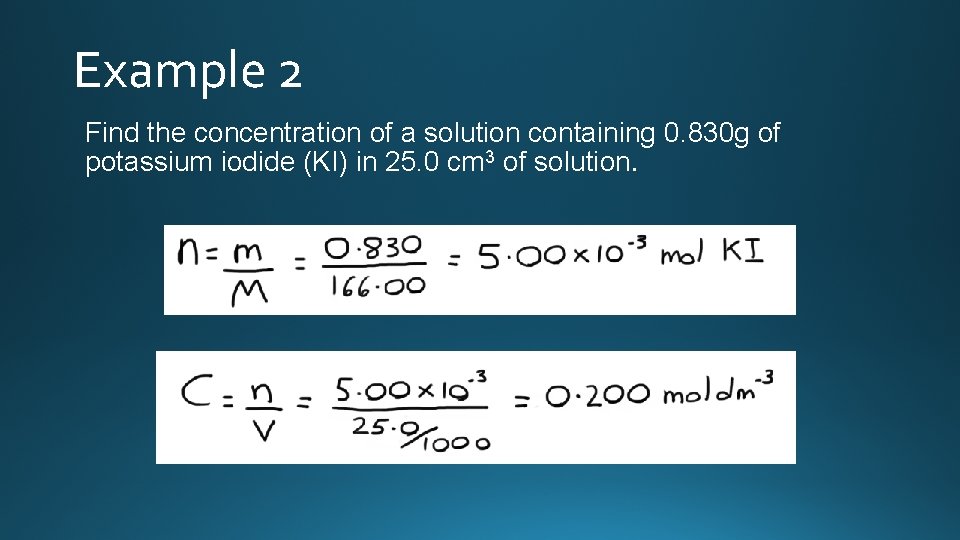
Example 2 Find the concentration of a solution containing 0. 830 g of potassium iodide (KI) in 25. 0 cm 3 of solution.
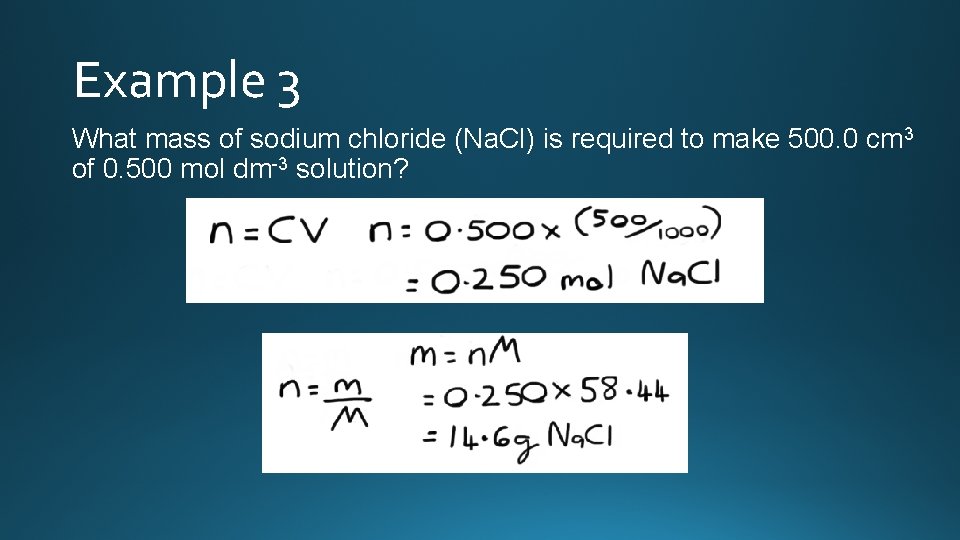
Example 3 What mass of sodium chloride (Na. Cl) is required to make 500. 0 cm 3 of 0. 500 mol dm-3 solution?

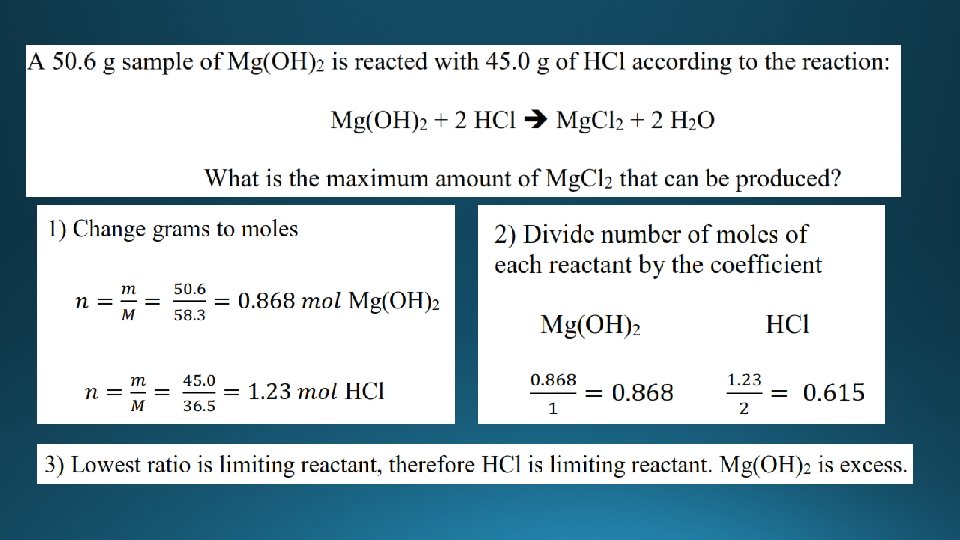
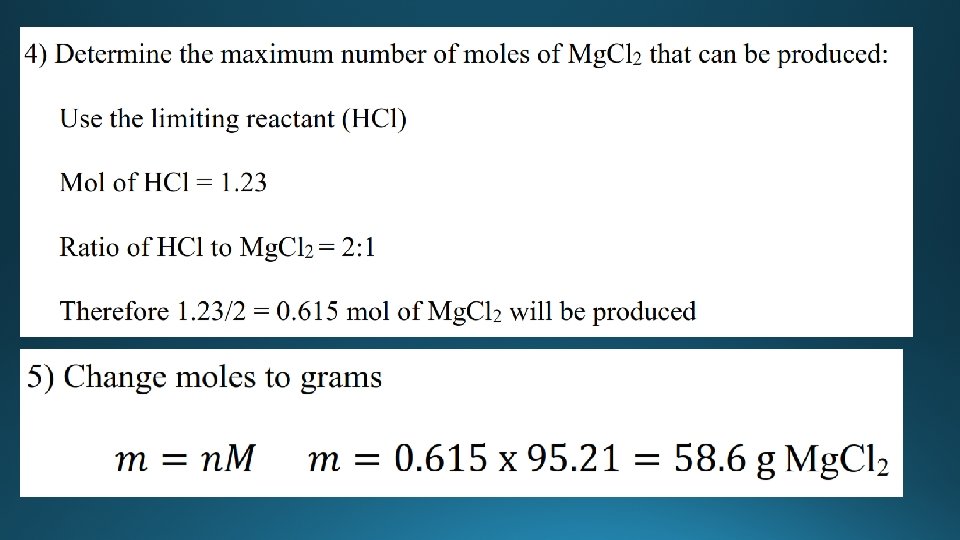
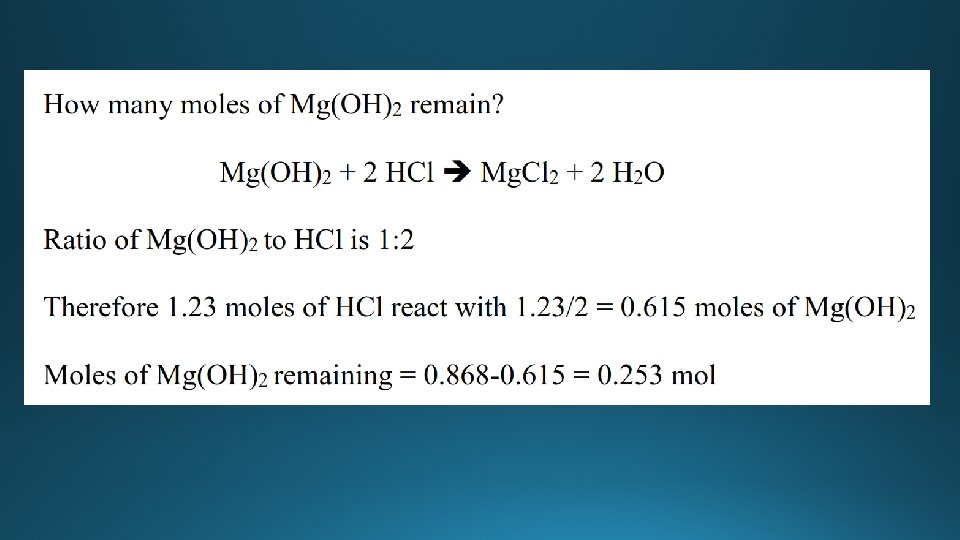
Limiting reactant • The limiting reactant (reagent) is the reactant that is completely used up during a chemical reaction. • The reactant that is in excess is the reactant that is not completely used up during the chemical reaction - there is some of this reactant left over at the end of the reaction. • Wheels are the limiting reactant, car bodies are in excess.

- Slides: 45