QUANTITATIVE CHEMISTRY Chapter 1 MOLE CONCEPT AVOGADROS CONSTANT



















- Slides: 31

QUANTITATIVE CHEMISTRY Chapter 1

MOLE CONCEPT & AVOGADRO’S CONSTANT All units will be metric in nature Use SI system – System Internationale Volume – standard is m 3 but dm 3 and cm 3 are more commonly used in lab setting 1 L = 1 dm 3 1 m. L = 1 cm 3 Mass – standard is kg, more common to use grams in lab setting 1 kg = 2. 2 lbs 1 lb = 454 g

MOLE CONCEPT & AVOGADRO’S CONSTANT If a substance is an atom we count atoms of it. If a substance is a compound we usually count molecules of it. Mole (mol) – amount of a substance that contains the same number of chemical species as there are in exactly 12 grams of the isotope carbon-12.

MOLE CONCEPT & AVOGADRO’S CONSTANT Relative atomic mass (Ar) – average atomic mass of the element, taking into account all its isotopes and their relative abundance, compared to one atom of carbon-12. Molar mass (M) – the mass of one mole of a species. It is the mass expressed in grams and has units of g mol-1. All molar masses will be expressed and rounded, if necessary, to two numbers after the decimal (16. 00 would not be rounded to 16).

MOLE CONCEPT & AVOGADRO’S CONSTANT Molecular formula – composition of a molecule (elements that make up the compound). Relative molecular mass (Mr) – sum of the relative atomic masses of the atoms in the molecular formula. Example: C 6 H 5 OH

MOLE CONCEPT & AVOGADRO’S CONSTANT If you are dealing with an ionic compound we must call it the relative formula mass (even though it’s really the same thing). Ionic compounds are made up of ions and generally start with a metal. Relative formula mass – sum of the relative atomic masses of the ions in a formula. Example: Ca(NO 3)2

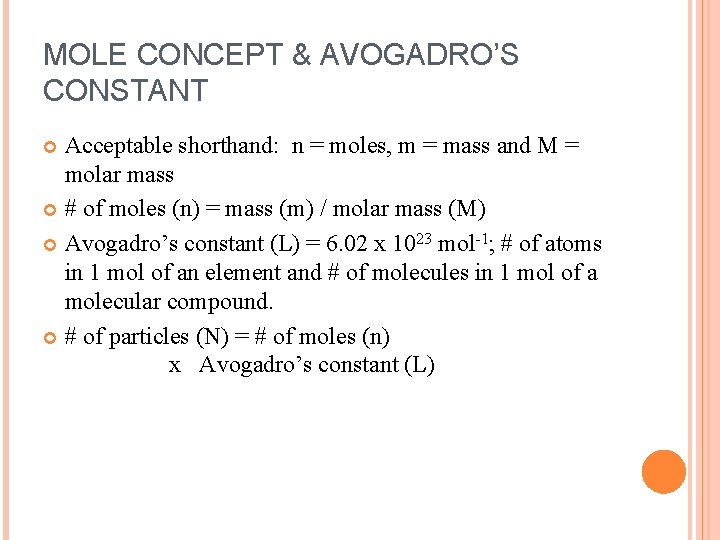
MOLE CONCEPT & AVOGADRO’S CONSTANT Acceptable shorthand: n = moles, m = mass and M = molar mass # of moles (n) = mass (m) / molar mass (M) Avogadro’s constant (L) = 6. 02 x 1023 mol-1; # of atoms in 1 mol of an element and # of molecules in 1 mol of a molecular compound. # of particles (N) = # of moles (n) x Avogadro’s constant (L)

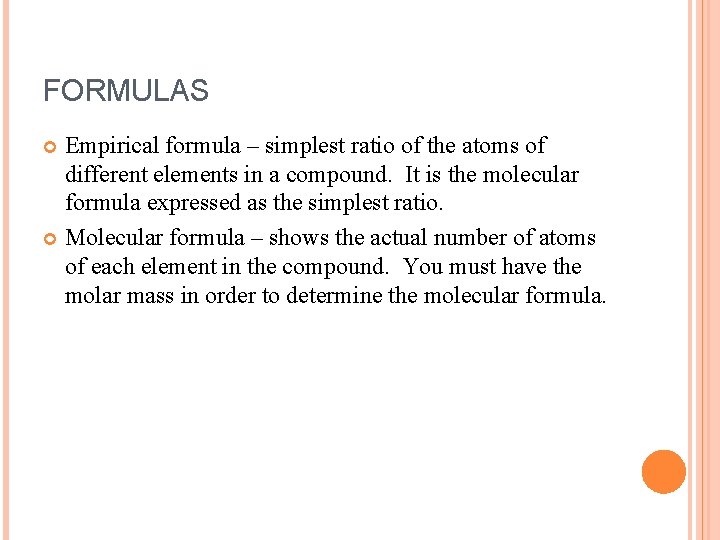
FORMULAS Empirical formula – simplest ratio of the atoms of different elements in a compound. It is the molecular formula expressed as the simplest ratio. Molecular formula – shows the actual number of atoms of each element in the compound. You must have the molar mass in order to determine the molecular formula.

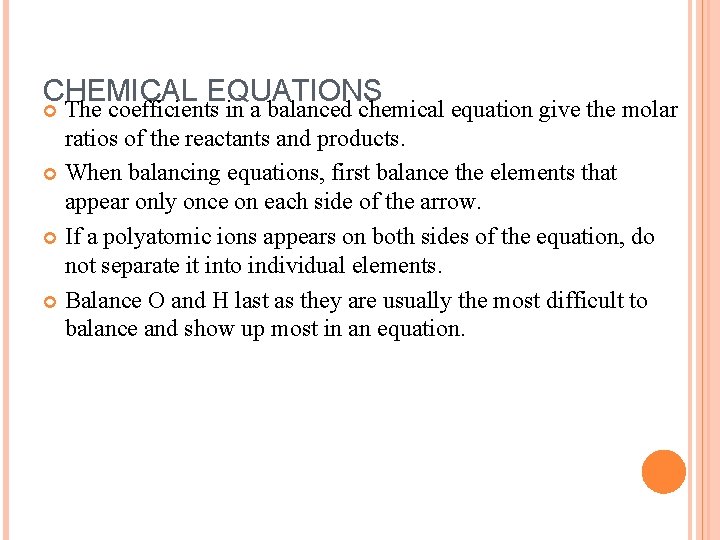
CHEMICAL EQUATIONS The coefficients in a balanced chemical equation give the molar ratios of the reactants and products. When balancing equations, first balance the elements that appear only once on each side of the arrow. If a polyatomic ions appears on both sides of the equation, do not separate it into individual elements. Balance O and H last as they are usually the most difficult to balance and show up most in an equation.

MASS AND GASEOUS VOLUME RELATIONSHIPS IN CHEMICAL REACTIONS Mass (m) = # of moles (n) x molar mass (M) In stoichiometry problems , follow these steps: 1. Convert grams to moles by dividing by M. 2. Convert moles of what you start with to moles of what you are trying to find using the coefficients of the balanced equation. 3. Convert moles of what you are trying to find back to grams by multiplying by the molar mass of what you are trying to find.

MASS AND GASEOUS VOLUME RELATIONSHIPS IN CHEMICAL REACTIONS Limiting reactant/reagent – reactant that is used up first in a chemical reaction. It is the substance that makes a smaller amount of product. Amount of product produced (theoretical yield) – comes from the limiting reactant and is the smaller of the two amounts that could be produced. It is the most product that could be produced from the reactants you start with.

MASS AND GASEOUS VOLUME RELATIONSHIPS IN CHEMICAL REACTIONS To find the amount of the excess reactant, subtract the amounts of the two products and convert the difference back to the excess reactant (reactant the made the larger amount of product). % Yield = Experimental (Actual) Yield x 100 Theoretical Yield

MASS AND GASEOUS VOLUME RELATIONSHIPS IN CHEMICAL REACTIONS Reasons % Yield may not be 100% Reaction is incomplete Side reaction occurring that is unwanted Complete separation of the product from the reaction mixture is impossible (usually in filtering) Product is lost during transfer process Mixture is not completely dried to evaporate off all of the water (% yield would be over 100%). Why is % yield an important concept in industry?

MASS AND GASEOUS VOLUME RELATIONSHIPS IN CHEMICAL REACTIONS Kinetic Theory has 2 basic ideas All matter consists of particles in motion As the temperature increases, the movement of particles increases The state of matter at a given temperature and pressure is determined by the strength of the interparticle forces.

MASS AND GASEOUS VOLUME RELATIONSHIPS IN CHEMICAL REACTIONS Solid – interparticle forces restrict movement to vibration about a fixed position. Liquid – interparticle forces are sufficiently weak to allow particles to change places with each other, but their movement is constrained to a fixed volume. Gas – interparticle forces are negligible.

MASS AND GASEOUS VOLUME RELATIONSHIPS IN CHEMICAL REACTIONS All temperatures in Kelvin (K = o. C + 273) Absolute zero – lowest temperature attainable and where all movement stops. Average kinetic energy is directly proportional to Kelvin temperature.

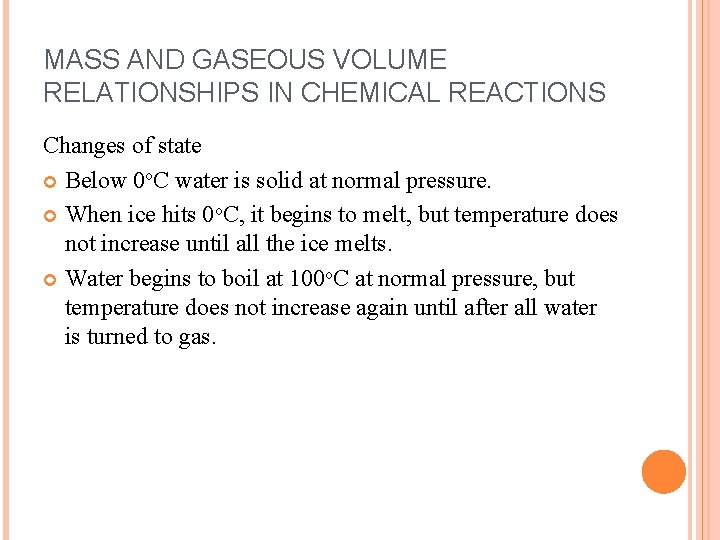
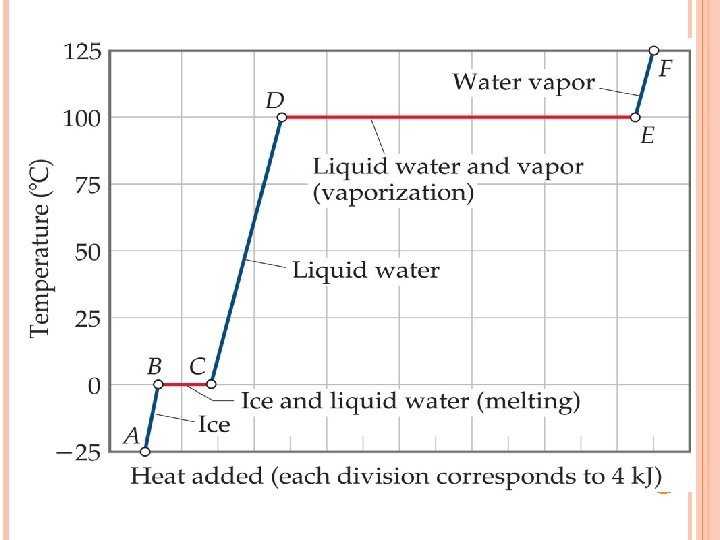
MASS AND GASEOUS VOLUME RELATIONSHIPS IN CHEMICAL REACTIONS Changes of state Below 0 o. C water is solid at normal pressure. When ice hits 0 o. C, it begins to melt, but temperature does not increase until all the ice melts. Water begins to boil at 100 o. C at normal pressure, but temperature does not increase again until after all water is turned to gas.


MASS AND GASEOUS VOLUME RELATIONSHIPS IN CHEMICAL REACTIONS Freezing – liquid to solid Melting – solid to liquid Boiling – liquid to gas Condensing – gas to liquid Deposition – gas directly to solid Sublimation – solid directly to gas

MASS AND GASEOUS VOLUME RELATIONSHIPS IN CHEMICAL REACTIONS At the melting point, the energy that is added is used to break the intermolecular bonds of the solid. At the boiling point, the energy that is added is used to break the intermolecular bonds of the liquid. Kinetic energy depends on mass (m) and speed (v). All gases at the same temperature have the same average kinetic energy. KE = ½ mv 2 , more massive particles at the same temperature have a lower speed to have equal

MASS AND GASEOUS VOLUME RELATIONSHIPS IN CHEMICAL REACTIONS Volatile substances have low boiling points, but are not necessarily flammable. Avogadro’s hypothesis – equal volume of different gases contain equal numbers of particles at the same temperature and pressure.

MASS AND GASEOUS VOLUME RELATIONSHIPS IN CHEMICAL REACTIONS Standard conditions or standard temperature and pressure (STP) – 273 K and 100 k. Pa, replaces previous standard pressure of 1 atm (101. 3 k. Pa). One mole of a gas at STP occupies 22. 4 dm 3 or 22, 400 cm 3 (22. 4 L). Number of moles (n) = volume (V) molar volume (Vmol)

MASS AND GASEOUS VOLUME RELATIONSHIPS IN CHEMICAL REACTIONS Gas Laws An increase in the frequency or energy of gas particle collisions will increase the pressure An increase in volume decreases frequency of collisions so pressure decreases (assuming Kelvin temp and amount of gas are constant. Pressure and volume are inversely proportional (P = k 1/V), k 1 is a constant. This is Boyle’s law.

MASS AND GASEOUS VOLUME RELATIONSHIPS IN CHEMICAL REACTIONS Pressure is directly proportional to Kelvin (absolute) temperature assuming volume and amount of gas are constant. P = k 2 T where k 2 is a constant, this is Charles’ law. Volume is directly proportional Kelvin temperature assuming pressure and amount of gas are constant. V = k 3 T where k 3 is constant, this is Gay-Lussac’s law.

MASS AND GASEOUS VOLUME RELATIONSHIPS IN CHEMICAL REACTIONS Combined Gas Law – combines Boyle’s, Charles’ and Gay-Lussac’s laws holding only the amount of a gas constant. P 1 V 1/T 1 = P 2 V 2/T 2

MASS AND GASEOUS VOLUME RELATIONSHIPS IN CHEMICAL REACTIONS Ideal Gas Law is PV = n. RT, where R is the ideal gas constant (8. 31 J K-1 mol-1) P should be in units of Pa (1 k. Pa = 1000 Pa) & V should be in units of m 3 (1 m 3 = 1000 dm 3) 1 atm = 101. 3 k. Pa = 760 mm Hg = 760 torr inches of Hg = 29. 92

MASS AND GASEOUS VOLUME RELATIONSHIPS IN CHEMICAL REACTIONS PV = n. RT can be used to find molar mass. n = m/M and can be rearranged in PV = n. RT to give M = m. RT/PV Density: r = m/V M = r. RT/P when density is in g m-3

SOLUTIONS Various containers can be used to measure volumes, including erlenmeyer flasks, beakers, graduated cylinders, volumetric flasks and burettes. Solutions are made up of a solute and a solvent. Solute – less abundant part of solution Solvent – more abundant part of solution, usually water if not specifically stated.

SOLUTIONS Composition of a solution is expressed in concentration (molarity). When solvent cannot hold any more solute it is said to be saturated. If any more solute is added it will not dissolve. Units for concentration are amount of solute in moles dissolved in 1 dm 3 of solvent, so units are mol dm-3 or g dm. Square brackets are used to represent concentration.

SOLUTIONS Standard solution – solution of known concentration. Dilution – standard solution that is diluted. # of moles = concentration x volume Concentration 1 x V 1 = Concentration 2 x V 2

SOLUTIONS Steps for Titration 1. A known volume of one solution is measured into a flask using a pipette. 2. The other solution is then added from a burette to find the equivalence point (volume when reaction is complete). In an acid-base titration, an indicator is used to determine when the equivalence point has been reached. The most common acid-base indicator is phenolphthalein.