Quantifying atoms and Molecules the relative isotopic masses



- Slides: 11

Quantifying atoms and Molecules • the relative isotopic masses of elements and their representation on the relative mass scale using the carbon-12 isotope as the standard; reason for the selection of carbon-12 as the standard • determination of the relative atomic mass of an element using mass spectrometry (details of instrument not required) • the mole concept; Avogadro constant; determination of the number of moles of atoms in a sample of known mass; calculation of the molar mass of ionic compounds • experimental determination of the empirical formula of an ionic compound.



Isotopes • Atoms of an element with the same number of protons (same atomic number) but different numbers of neutrons (different mass number) in the nucleus.

• the relative isotopic masses of elements and their representation on the relative mass scale using the carbon-12 isotope as the standard; reason for the selection of carbon-12 as the standard Relative masses The masses most frequently used in chemistry are relative masses, rather than actual masses. The standard to which all masses are compared is the mass of an atom of the common isotope of carbon, carbon 12 or 12 C which is given a mass of exactly 12.

• the relative isotopic masses of elements and their representation on the relative mass scale using the carbon-12 isotope as the standard; reason for the selection of carbon-12 as the standard • Carbon-12 was selected as the standard in 1961. Before this Oxygen was used as the standard but physicists and chemists could not agree on a way of assigning a standard mass to oxygen. Making Carbon-12 the standard, with 6 electrons and 6 protons, the standard and assigning it a mass of exactly 12 created a new scale, which was adopted universally.

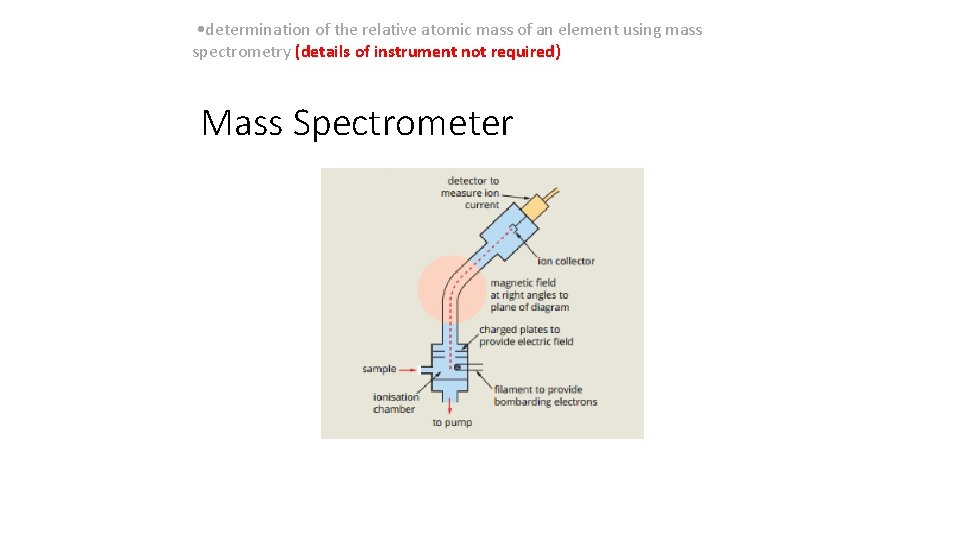
• determination of the relative atomic mass of an element using mass spectrometry (details of instrument not required) Mass Spectrometer • Currently most accurate way to evaluate the mass of an atom. • Atoms or molecules are passed into a beam of high speed electrons which coverts them into positive ions. • The positive ions are then accelerated through an electric field and deflected by a magnetic field. • The lightest and most highly charged element is deflected the most. • Eg: 63 Cu+is deflected more than 65 Cu+ • Eg: 63 Cu 2+is deflected more than 63 Cu+ • A mass spectrum shows information about the relative isotopic mass (Ir), percentage abundance of each isotope and the number of isotopes in a given sample.

• determination of the relative atomic mass of an element using mass spectrometry (details of instrument not required) Mass Spectrometer

• the relative isotopic masses of elements and their representation on the relative mass scale using the carbon-12 isotope as the standard; reason for the selection of carbon-12 as the standard Relative Isotopic Mass (Ir) • Defined as “The mass of an individual isotope of an element on the relative atomic mass scale, on which the masses of particles are compared with the mass of the carbon-12 isotope. ” • All other isotopic masses are measured relative to carbon 12. • Masses of other isotopes and atoms are determined in mass spectrometers. • Since masses are relative, relative isotopic mass has no units.

Relative Atomic Mass (Ar) • The relative atomic mass of an element represents the weighted average of the relative isotopic masses of all the naturally occurring isotopes of a sample of an element relative to carbon-12 which is assigned a mass of exactly 12. It takes into consideration the relative abundance of each isotope. • As masses are relative to carbon-12 Ar has no units. • Relative atomic mass data can be found on your periodic table. • Can be calculated using this formula: • Ar = (Ir of 1 st isotope x % abundance) + (Ir of 2 nd isotope x % abundance). . . 100
Relative molecular mass and Relative formula mass (Mr ) • Relative molecular mass: the mass of a molecule relative to carbon-12. Applies only to molecular substances. • Relative formula mass: the sum of the relative atomic masses of the atoms as given in the chemical formula of a species. Applies to molecular and non-molecular substances such as ionic solids. • Calculated by adding up the atomic masses of the atoms as given by the formula of the substance. • Eg: Mr(H 2 O) = 2 x Ar(H)+ Ar(O) = 2 x 1. 0 + 16. 0 = 18. 0