Quality Management System Quality Management System QMS Requirements











- Slides: 15

Quality Management System

Quality Management System QMS Requirements Ø QMS Integration / Development Ø QMS Processing Ø QMS Summary Ø

QMS Requirements

QMS Requirements Ø Ability to Create, Modify, and Store Inspection Plans (Specifications, Samples, Tests) within BPCS Ø Ability to keep past versions of Inspection Tests and Descriptions Ø Ability to assign Inspection Specifications to each item / manufacturer Ø Ability to inspect and record results for both Lot controlled items and non-Lot controlled items (sequence) Ø Ability to Snap-Shot the Specification (Samples, Tests) for each item / lot or item / sequence

QMS Requirements Ø Ability to perform inspections and record results as product is being assembled Ø Ability to enter Notes for each Test during results recording and ability to append to those notes while protecting previous notes Ø Ability to create ad-hoc Tests during inspection Ø Ability for extensive Reporting Ø Ability to handle Non-Conformance Reporting

QMS Integration / Development

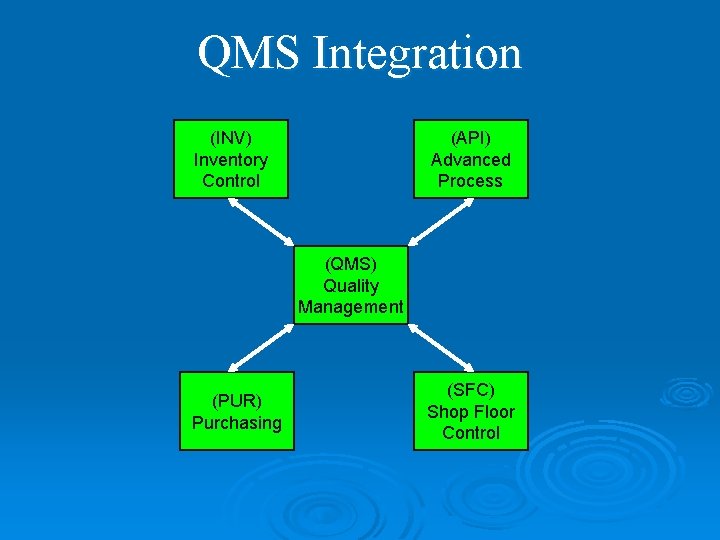
QMS Integration (INV) Inventory Control (API) Advanced Process (QMS) Quality Management (PUR) Purchasing (SFC) Shop Floor Control

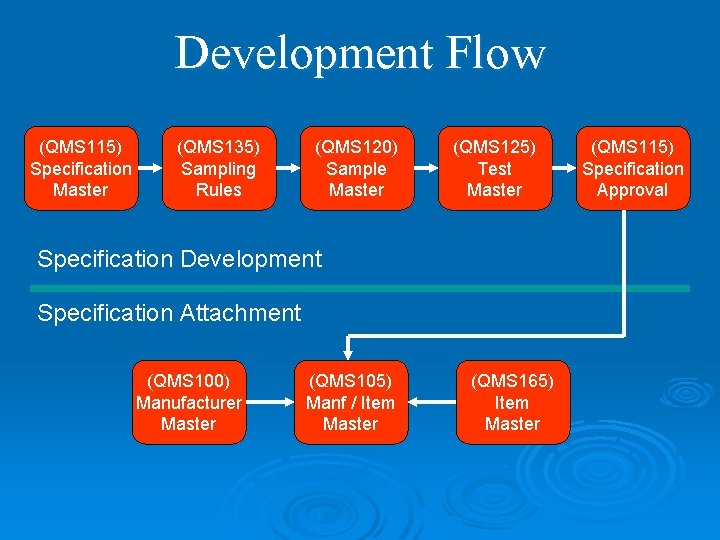
Development Flow (QMS 115) Specification Master (QMS 135) Sampling Rules (QMS 120) Sample Master (QMS 125) Test Master Specification Development Specification Attachment (QMS 100) Manufacturer Master (QMS 105) Manf / Item Master (QMS 165) Item Master (QMS 115) Specification Approval

QMS Processing

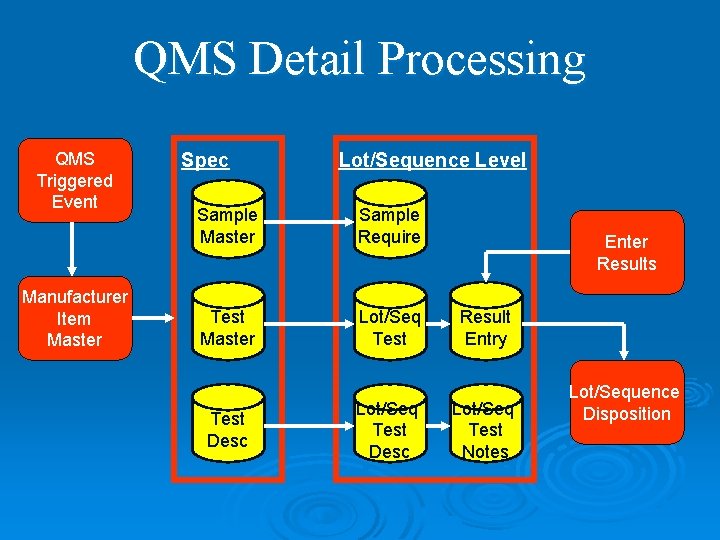
QMS Detail Processing QMS Triggered Event Manufacturer Item Master Spec Lot/Sequence Level Sample Master Sample Require Test Master Lot/Seq Test Desc Enter Results Result Entry Lot/Seq Test Notes Lot/Sequence Disposition

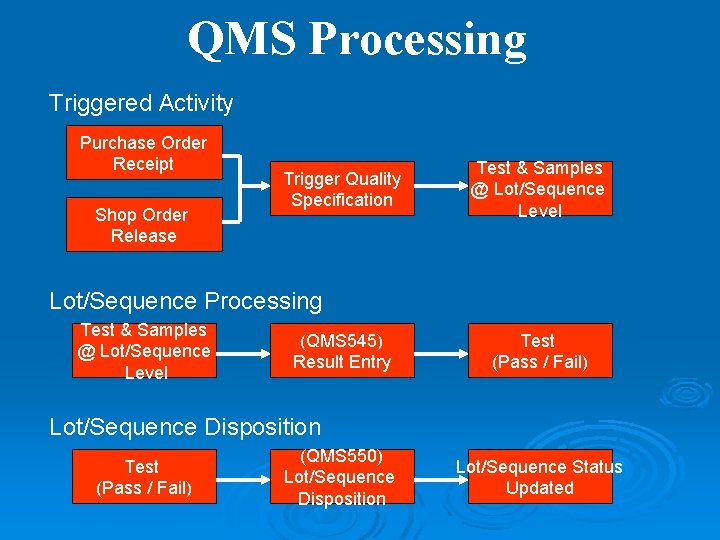
QMS Processing Triggered Activity Purchase Order Receipt Shop Order Release Trigger Quality Specification Test & Samples @ Lot/Sequence Level Lot/Sequence Processing Test & Samples @ Lot/Sequence Level (QMS 545) Result Entry Test (Pass / Fail) Lot/Sequence Disposition Test (Pass / Fail) (QMS 550) Lot/Sequence Disposition Lot/Sequence Status Updated

Incoming Inspection QMS Triggered by PO Receipt (PUR 550) Ø Item Lot/Sequence Inspection Created at Receipt l Item Lot/Sequence Status “Q” Ø Specification Plan Launched l Based on Manufacturer (Vendor) / Item Ø Ø Inspection and Results recording Ø Disposition (Accept = Status “A” Reject = Status “R”)

In-Process Inspection QMS Triggered by Shop Order Release Ø Item Lot/Sequence Inspection Created at Release l Item Lot/Sequence Status “Q” Ø Specification Plan Launched l Based on Manufacturer / Item Ø Ø Inspection and Results recording Ø Disposition (Accept = Status “A” Reject = Status “R”)

QMS Summary

Advantages of using QMS Improved inventory control Ø Central control of sample and test records Ø Central control over quality documentation Ø On line visibility of data and documents Ø Central Reporting and Traceability Ø All within the BPCS System Ø