Quality Improvement Measuring Quality of Care with Electronic









![e. CQI Resource Center www. healthit. gov/ecqi-resource-center e. CQI Resource Center [Screen shot]. Retrieved e. CQI Resource Center www. healthit. gov/ecqi-resource-center e. CQI Resource Center [Screen shot]. Retrieved](https://slidetodoc.com/presentation_image/bee03acef5204c5a79044c8cc25f5f69/image-15.jpg)
![USHIK: United States Health Information Knowledgebase USHIK [Screen shot]. Retrieved March 2012, from https: USHIK: United States Health Information Knowledgebase USHIK [Screen shot]. Retrieved March 2012, from https:](https://slidetodoc.com/presentation_image/bee03acef5204c5a79044c8cc25f5f69/image-16.jpg)





- Slides: 24

Quality Improvement Measuring Quality of Care with Electronic Clinical Quality Measures (e. CQMs) This material (Comp 12 Unit 10) was developed by Johns Hopkins University, funded by the Department of Health and Human Services, Office of the National Coordinator for Health Information Technology under Award Number IU 24 OC 000013. This material was updated in 2016 by Johns Hopkins University under Award Number 90 WT 0005. This work is licensed under the Creative Commons Attribution-Non. Commercial-Share. Alike 4. 0 International License. To view a copy of this license, visit http: //creativecommons. org/licenses/by-nc-sa/4. 0/.

Measuring Quality of Care with Electronic Clinical Quality Measures (e. CQM) Learning Objectives • Review types of quality and safety measures currently in use nationally. • Explain the attributes of an effective electronic clinical quality measures (e. CQMs) reporting system. • Examine the importance of having standardized and structured health information for quality measurement, especially electronic clinical quality measures (e. CQMs). • Discuss the role of HIT standards and terminologies in electronic clinical quality measures. • Discuss how HIT can facilitate data collection and reporting for improving quality of care and patient safety. • Describe data quality issues in electronic measures. 2

Understanding Different Types of Quality Measurement • Based on data sources and types of specifications, the following types of quality measurement are currently in use: – Claims. – Abstraction. – Electronic (e. CQM). 3

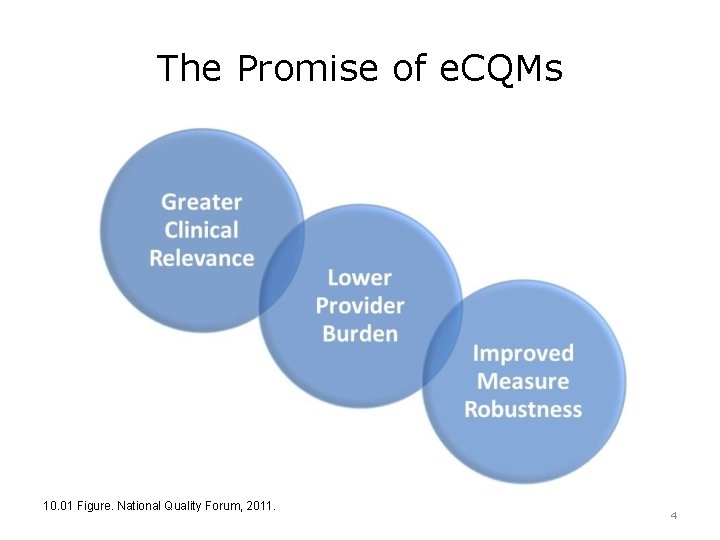
The Promise of e. CQMs 10. 01 Figure. National Quality Forum, 2011. 4

e. CQM Lifecycle 10. 02 Figure. 5

Chart Abstraction vs. e. CQM: Measurement Specification Development Process • Paper-based measure development: – – – Develop measure narrative, numerator/denominator in line with existing administrative data and/or data typically found in patient medical records (these can be paper or electronic charts). Create a list of code sets, data elements, and abstraction definitions to represent the concepts within the measure. Solicit public comment on the measures (program specific). Measure developers conduct complete feasibility, reliability, and validity testing. Measures for use in national programs are generally submitted to the NQF for endorsement. • e. CQM measure development: – – – – Develop measure narrative, numerator/ denominator, workflow, and logic, in line with existing standards (e. g. , Blueprint for the CMS Measures Management System [e. Measure Specifications section], Quality Data Model [QDM]). Create value sets, collaborating with the Value Set Authority Center and clinical terminology (e. g. , SNOMED-CT, LOINC) stakeholders, as needed. Use the Measure Authoring Tool (MAT) to create the e. CQM in Health Quality Measure Format (HQMF). Conduct complete feasibility, reliability, and validity testing, which can include working with EHR vendors to understand data element availability and implementation in the field. Develop the implementation test decks or test cases for the Cypress certification tool. Collaborate with other stakeholders (e. g. , Health Level 7 [HL 7], the e. Measures Issues Group [e. MIG]). Solicit public comment on the measures (program specific). 6

e. CQM Specifications • XML: • Human-readable: – Description: a CQM written in – Description: the human- Health Quality Measures Format (HQMF) syntax. HQMF is the industry (HL 7) standard for representing a CQM as an electronic document. – Likely user: EHR system developers and administrators, analysts. – Use: to enable the automated creation of queries against an EHR or other operational data store for quality reporting. readable HTML equivalent of the XML file content. – Likely user: EHR users. – Use: to identify the details of the CQM in a human-readable format, so that the user can understand both how the elements are defined and the underlying logic of the measure calculation. 7

HQMF: Human-Readable Excerpt from e. CQM Specifications Human Readable file, retrieved March 2012 from CMS’s EHR Incentive Programs e. CQM Library: http: //www. cms. gov/Regulations-and-Guidance/Legislation/EHRIncentive. Programs/e. CQM_Library. html 8

HQMF: Machine-Readable XML Excerpt from e. CQM Specifications Machine Readable file, retrieved March 2012 from CMS’s EHR Incentive Programs e. CQM Library: http: //www. cms. gov/Regulations-and-Guidance/Legislation/EHRIncentive. Programs/e. CQM_Library. html 9

e. CQM Standards – 1 • Measure specification standards: – Quality Data Model (QDM). – Health Quality Measure Format (HQMF). • Measure results reporting standards: – Quality Reporting Document Architecture (QRDA). – Category I for patient-level data. – Category III for aggregate data. 10

e. CQM Standards – 2 To view e. CQM packages: http: //www. cms. gov/Regulations-and. Guidance/Legislation/EHRIncentive. Programs/e. CQM_Library. html 10. 03 Figure. 11

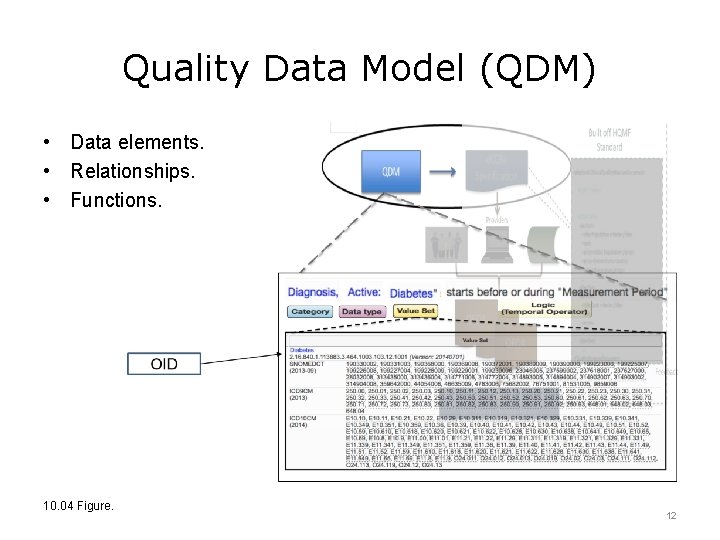
Quality Data Model (QDM) • Data elements. • Relationships. • Functions. 10. 04 Figure. 12

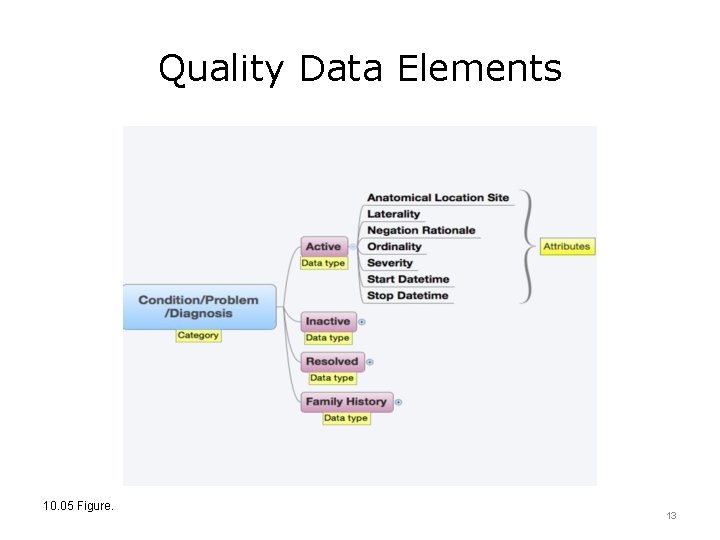
Quality Data Elements 10. 05 Figure. 13

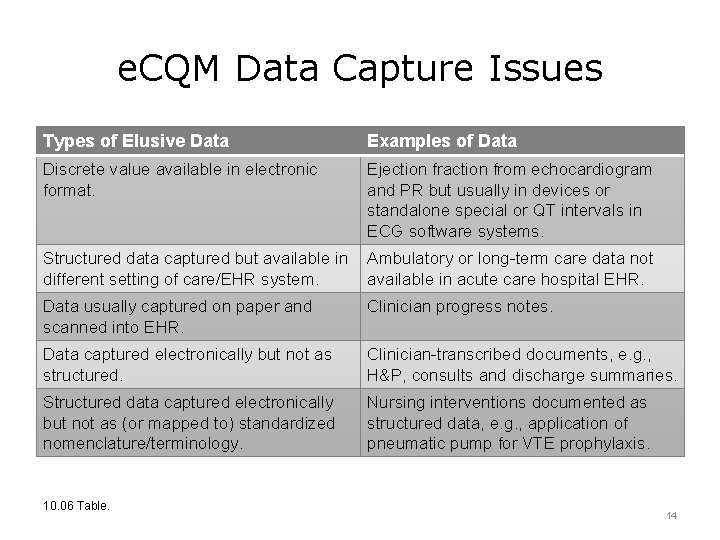
e. CQM Data Capture Issues Types of Elusive Data Examples of Data Discrete value available in electronic format. Ejection fraction from echocardiogram and PR but usually in devices or standalone special or QT intervals in ECG software systems. Structured data captured but available in Ambulatory or long-term care data not different setting of care/EHR system. available in acute care hospital EHR. Data usually captured on paper and scanned into EHR. Clinician progress notes. Data captured electronically but not as structured. Clinician-transcribed documents, e. g. , H&P, consults and discharge summaries. Structured data captured electronically but not as (or mapped to) standardized nomenclature/terminology. Nursing interventions documented as structured data, e. g. , application of pneumatic pump for VTE prophylaxis. 10. 06 Table. 14
![e CQI Resource Center www healthit govecqiresourcecenter e CQI Resource Center Screen shot Retrieved e. CQI Resource Center www. healthit. gov/ecqi-resource-center e. CQI Resource Center [Screen shot]. Retrieved](https://slidetodoc.com/presentation_image/bee03acef5204c5a79044c8cc25f5f69/image-15.jpg)
e. CQI Resource Center www. healthit. gov/ecqi-resource-center e. CQI Resource Center [Screen shot]. Retrieved March 2012, from https: //ecqi. healthit. gov/ecqm 15
![USHIK United States Health Information Knowledgebase USHIK Screen shot Retrieved March 2012 from https USHIK: United States Health Information Knowledgebase USHIK [Screen shot]. Retrieved March 2012, from https:](https://slidetodoc.com/presentation_image/bee03acef5204c5a79044c8cc25f5f69/image-16.jpg)
USHIK: United States Health Information Knowledgebase USHIK [Screen shot]. Retrieved March 2012, from https: //ushik. ahrq. gov/Quality. Measures. Listing? draft=true&system=dcqm&sort. Field=570&sort. Direction=ascendi ng&enable. Asynchronous. Loading=true 16

e. CQM Implementation Team • • Project manager. Quality director/manager. HIT analyst. Database analyst. Vendor support representative. SME consultant. Physician representative. Nurses representative. 17

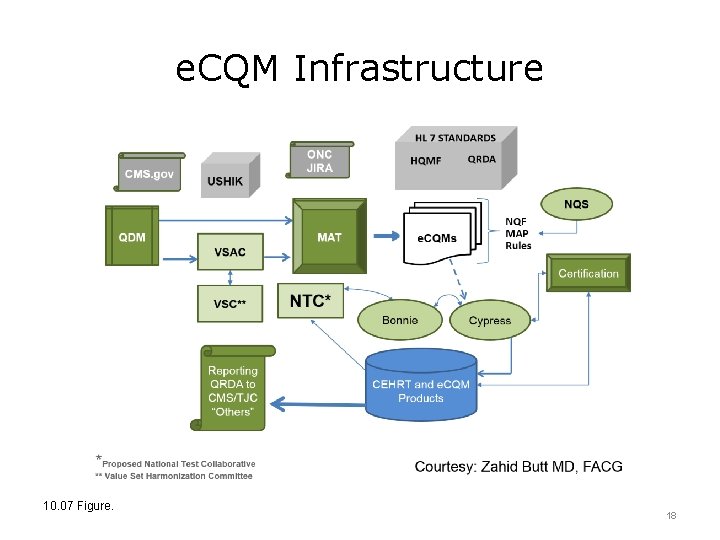
e. CQM Infrastructure 10. 07 Figure. 18

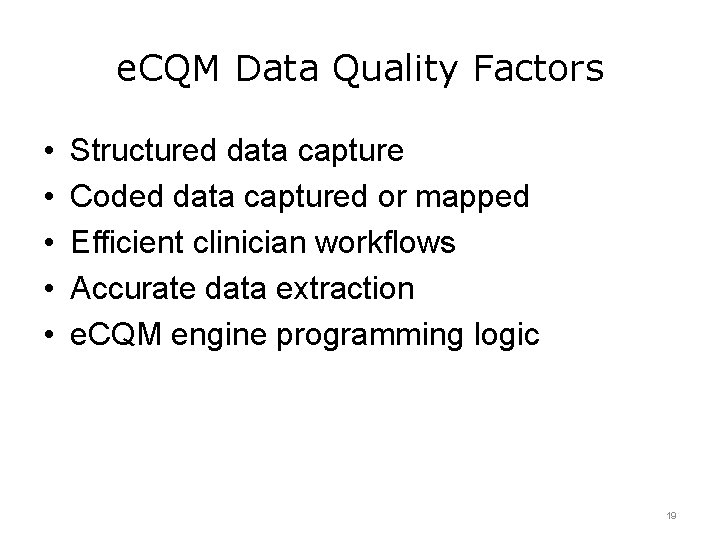
e. CQM Data Quality Factors • • • Structured data capture Coded data captured or mapped Efficient clinician workflows Accurate data extraction e. CQM engine programming logic 19

Measuring Quality of Care with Electronic Clinical Quality Measures (e. CQMs) Summary • Measurement is an essential component of quality improvement. • HIT has an important role to play in any measurement strategy. • e. CQMs are designed specifically to maximally leverage HIT and have the potential to revolutionize quality measurement. 20

Measuring Quality of Care with Electronic Clinical Quality Measures (e. CQM) References — 1 References Agency for Healthcare Research and Quality. (20 xx). United States health information knowledgebase. Retrieved March 2012, from http: //www. ushik. org/ Centers for Medicare & Medicaid Services (20 xx). e. CQM specifications human readable file. EHR Incentive Programs e. CQM Library. Retrieved March 2012, from http: //www. cms. gov/Regulations-and. Guidance/Legislation/EHRIncentive. Programs/e. CQM_Library. html Centers for Medicare & Medicaid Services (20 xx). e. CQM specifications machine readable file. EHR Incentive Programs e. CQM Library. Retrieved March 2012, from http: //www. cms. gov/Regulations-and. Guidance/Legislation/EHRIncentive. Programs/e. CQM_Library. html Centers for Medicare & Medicaid Services. (2016). Blueprint for the CMS measures management system. Version 12. 0. Retrieved May 31, 2016, from https: //www. cms. gov/Medicare/Quality-Initiatives-Patient-Assessment. Instruments/MMS/Downloads/Blueprint-120. pdf e. CQI Resource Center. (20 xx). Retrieved March 2012, from http: //www. healthit. gov/ecqiresource-center National Quality Forum. (2011). Electronic measures. Retrieved May 31, 2016, from http: //www. qualityforum. org/Projects/e 21 g/e. Measures/Electronic_Quality_Measures_(e. Measures). aspx

Measuring Quality of Care with Electronic Clinical Quality Measures (e. CQM) References — 2 Charts, Tables, Figures 10. 01 Figure: The Promise of e. CQMs. National Quality Forum. (2011). Electronic measures. Retrieved May 31, 2016, from http: //www. qualityforum. org/Projects/eg/e. Measures/Electronic_Quality_Measures_(e. Measures). aspx 10. 02 Figure: e. CQM Lifecycle. 10. 03 Figure: e. CQM Standards. 10. 04 Figure: Quality Data Model (QDM). 10. 05 Figure: Quality Data Elements. 10. 06 Table: e. CQM Data Capture Issues. 10. 07 Figure: e. CQM Infrastructure. Courtesy Zahid Butt, MD, FACG. 22

Measuring Quality of Care with Electronic Clinical Quality Measures (e. CQM) References — 3 Images Slide 8: HQMF: Human-Readable [Screen shot]. Retrieved March 2012, from http: //www. cms. gov/Regulations-and. Guidance/Legislation/EHRIncentive. Programs/e. CQM_Library. html Slide 9: HQMF Machine-Readable XML [Screen shot]. Retrieved March 2012, from http: //www. cms. gov/Regulations-and. Guidance/Legislation/EHRIncentive. Programs/e. CQM_Library. html Slide 15: e. CQI Resource Center [Screen shot]. Retrieved March 2012, from http: //www. healthit. gov/ecqi-resource-center Slide 16: United States Health Information Knowledgebase [Screen shot]. Retrieved March 2012, from http: //www. ushik. org/ 23

Quality Improvement Measuring Quality of Care with Electronic Clinical Quality Measures (e. CQM) This material (Comp 12 Unit 10) was developed by Johns Hopkins University, funded by the Department of Health and Human Services, Office of the National Coordinator for Health Information Technology under Award Number IU 24 OC 000013. This material was updated in 2016 by Johns Hopkins University under Award Number 90 WT 0005. 24
 Pocqi ppt
Pocqi ppt Ana model of quality assurance
Ana model of quality assurance Compliance vs quality
Compliance vs quality Small track machine manual
Small track machine manual Levels of nursing care primary secondary tertiary
Levels of nursing care primary secondary tertiary An electronic is the electronic exchange of money or scrip
An electronic is the electronic exchange of money or scrip Electronic field production
Electronic field production Qi 101: introduction to health care improvement
Qi 101: introduction to health care improvement Jhm irb
Jhm irb Qsen competencies safety examples
Qsen competencies safety examples Quality improvement paradigm
Quality improvement paradigm Define continuous quality improvement
Define continuous quality improvement Define continuous quality improvement
Define continuous quality improvement Efmd quality improvement system
Efmd quality improvement system Indiana perinatal quality improvement collaborative
Indiana perinatal quality improvement collaborative Cqi action plan template
Cqi action plan template Tea quality improvement
Tea quality improvement Xerox problem solving process
Xerox problem solving process Sustainability in quality improvement
Sustainability in quality improvement Cotinuous
Cotinuous Quality improvement
Quality improvement Juran 10 steps to quality improvement
Juran 10 steps to quality improvement Swot analysis for quality
Swot analysis for quality Mqii toolkit
Mqii toolkit Quality improvement
Quality improvement