Quality control of staining solution and reagents Mohammad














- Slides: 22

Quality control of staining, solution and reagents Mohammad Ali Jalali Far

Ø (3) The medical test site must maintain documentation of: q (a) Expiration date, lot numbers, and other pertinent information for: (i) Reagents; (ii) Solutions; (iii) Culture media; (iv) Controls; (v) Calibrators; (vi) Standards; (vii) Reference materials; and (viii) Other testing materials; and q (b) Testing of quality control samples.

Ø (4) For quantitative tests, the medical test site must perform quality control as follows: (a) Include two reference materials of different concentrations each day of testing unknown samples, if these reference materials are available; or (b) Follow an equivalent quality testing procedure that meets federal CLIA regulations. Ø (5) For qualitative tests, the medical test site must perform quality control as follows: (a) Use positive and negative reference material each day of testing unknown samples; or (b) Follow an equivalent quality testing procedure that meets federal CLIA regulations.

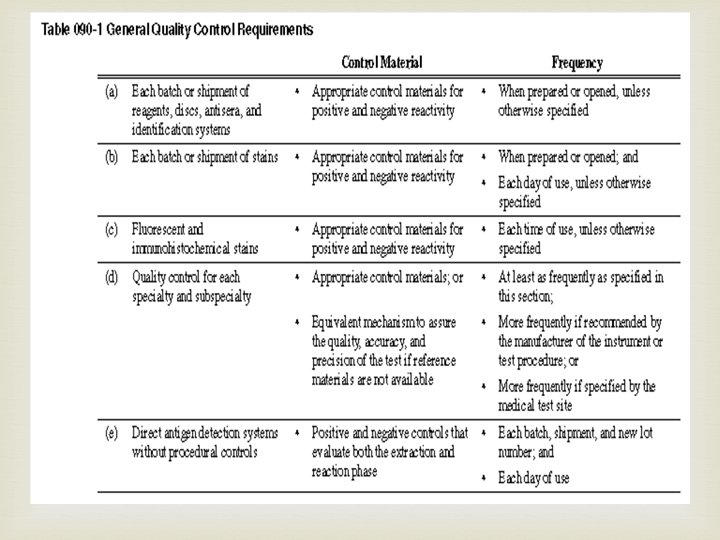
Ø (6) The medical test site must: (a) Use materials within their documented expiration date; (b) Not interchange components of kits with different lot numbers, unless specified by the manufacturer; (c) Determine the statistical limits for each lot number of unassayed reference materials through repeated testing; (d) Use the manufacturer's reference material limits for assayed material, provided they are: (i) Verified by the medical test site; and (ii) Appropriate for the methods and instrument used by the medical test site; (e) Make reference material limits readily available; (f) Report patient results only when reference materials are within acceptable limits; and (g) Rotate control material testing among all persons who perform the test; (h) Use calibration material from a different lot number than that used to establish a cut-off value or to calibrate the test system, if using calibration material as a control material; and (i) Comply with general quality control requirements as described in Table 090 -1, unless otherwise specified in subsection (9)(a) through (l) of this section.


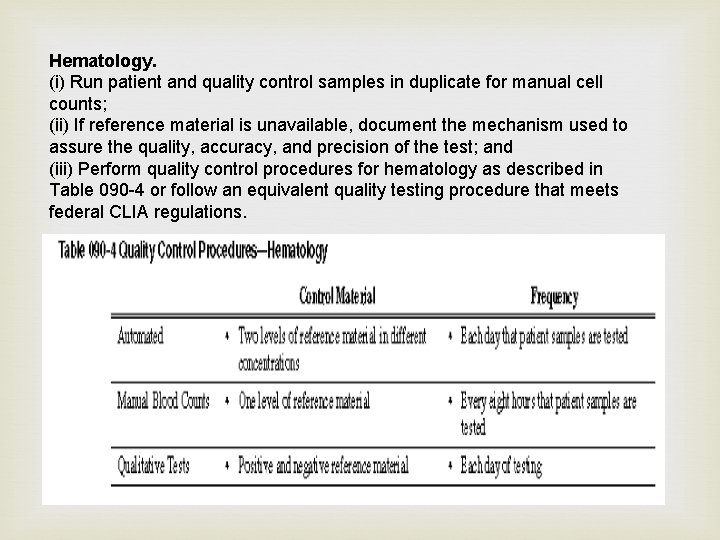
Hematology. (i) Run patient and quality control samples in duplicate for manual cell counts; (ii) If reference material is unavailable, document the mechanism used to assure the quality, accuracy, and precision of the test; and (iii) Perform quality control procedures for hematology as described in Table 090 -4 or follow an equivalent quality testing procedure that meets federal CLIA regulations.

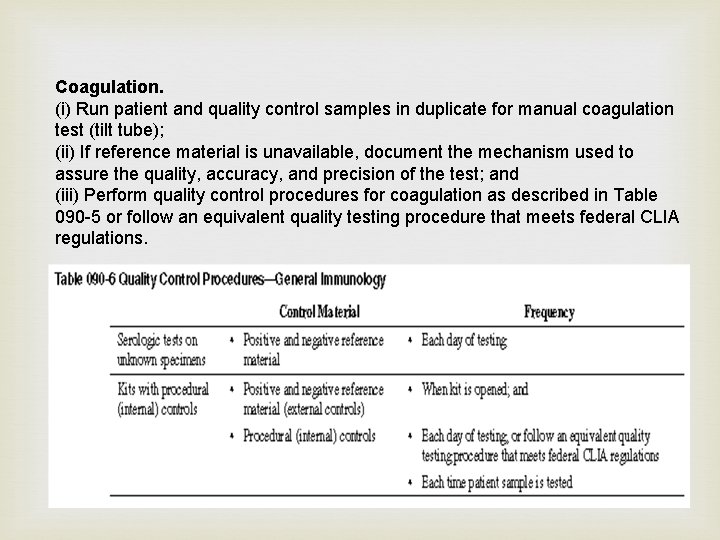
Coagulation. (i) Run patient and quality control samples in duplicate for manual coagulation test (tilt tube); (ii) If reference material is unavailable, document the mechanism used to assure the quality, accuracy, and precision of the test; and (iii) Perform quality control procedures for coagulation as described in Table 090 -5 or follow an equivalent quality testing procedure that meets federal CLIA regulations.

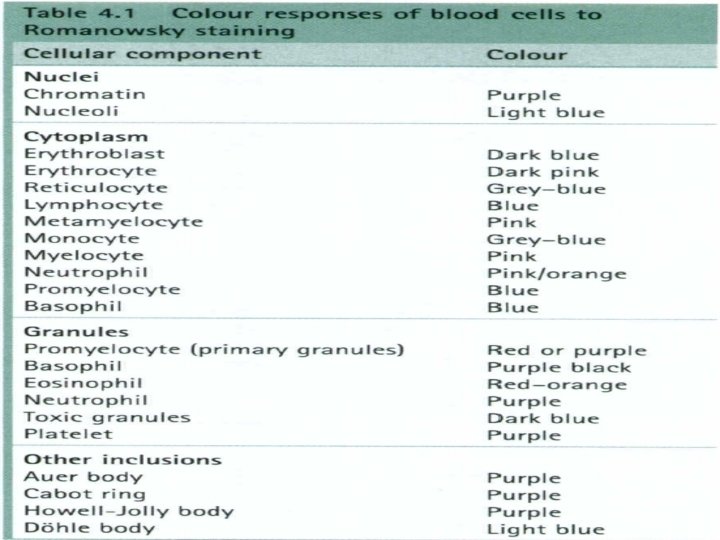
The common elements for QC are the same: the stains should be prepared and stored properly, and checked to be sure they perform as expected. Remember that many of the microscopic examinations that rely on stains are critical in diagnosis of many diseases. Stain management Some stains can be purchased commercially, but others must be prepared by the laboratory, following an established procedure. Once stains are made, their bottles should be labeled with the following information: Ø • name of the stain Ø • concentration Ø • date prepared Ø • date placed in service Ø • expiration date/shelf life Ø • preparer’s initials.

It may be useful to keep a log book for recording information on each stain in use, including the lot number and date received. The expiration date must be noted on the label. Some stains deteriorate and lose their ability to produce the correct reactions. Stains should be stored at the correct temperature at all times and in an appropriate staining bottle. Some stains must be protected from light. In some cases, working solutions can be made from stock solutions. If so, storage of working solutions should be carefully monitored.

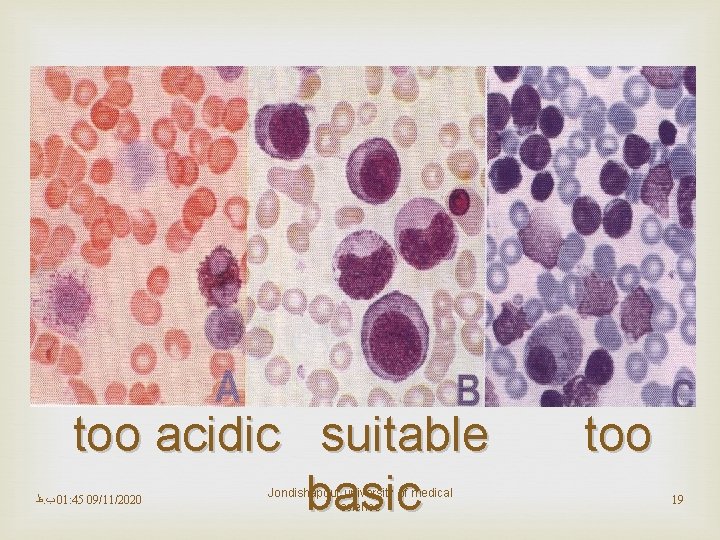
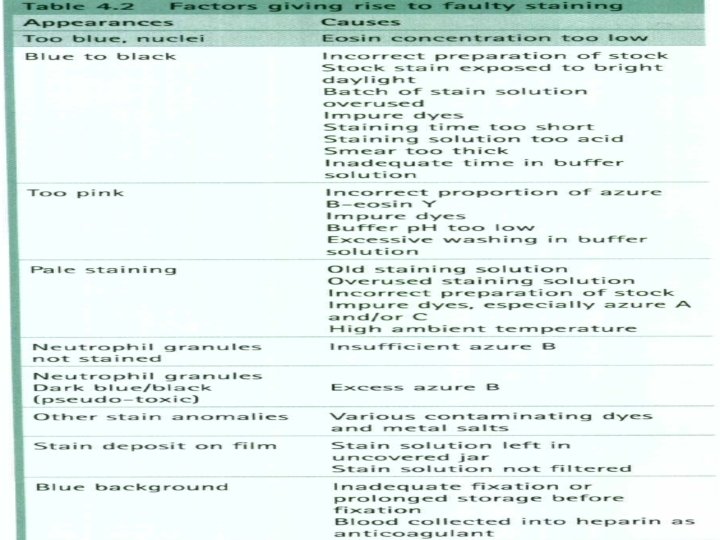
Quality control Because of their importance, stains should be checked each day of use with positive and negative QC materials, to make sure their reagents are active and they provide the intended results. In most cases, positive and negative controls should be stained with each batch of patients’ slides. All quality control results must be recorded each time they are run. Stains should also be examined to look for precipitation or crystal formation, and to check for bacterial contamination. Careful maintenance and care of the stock and working solutions of stains is an essential component in a system to provide good quality in microscopic examinations. Be aware that many stains are toxic therefore take appropriate safety precautions when working with them.

Key messages All staff must follow the quality control practices and procedures. • Always record quality control results and any corrective actions that are taken. • If QC results are not acceptable, do not report patient results.








too acidic suitable basic ﻅ. ﺏ 01: 45 09/11/2020 Jondishapour university of medical science too 19


