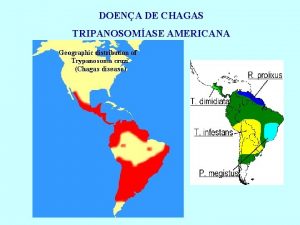
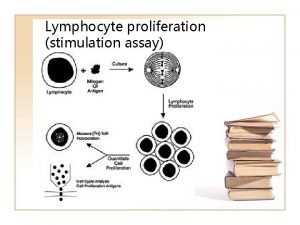
Quality Control of Chagas diagnostics immunoassays Assay characteristics











- Slides: 24

Quality Control of Chagas diagnostics immunoassays: Assay characteristics and manufacturer’s reference panels. Gustavo A. Capriotti, Biochemist, R&D Manager WHO Consultation Meeting 27 -28 January 2009

Antigens used in Conventional Tests for T. cruzi infection: ü Whole Extracts or Semipurified Fractions of parasite (epimastigote) ü Purified Proteins ü Synthetic Peptides ü Recombinant Antigens WHO Consultation Meeting 27 -28 January 2009

Conventional serological tests • Indirect hemagglutination (IHA) § Parasite lysate • ELISA § Parasite lysate § Recombinant antigens WHO Consultation Meeting 27 -28 January 2009

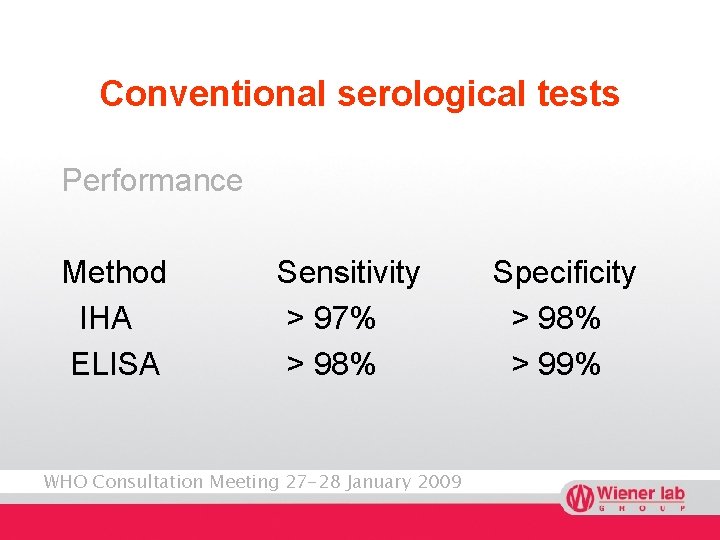
Conventional serological tests Performance Method IHA ELISA Sensitivity > 97% > 98% WHO Consultation Meeting 27 -28 January 2009 Specificity > 98% > 99%

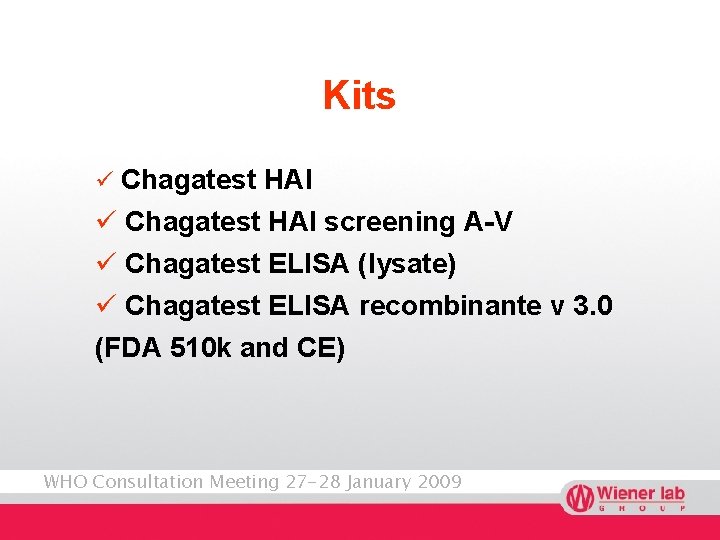
Kits ü Chagatest HAI screening A-V ü Chagatest ELISA (lysate) ü Chagatest ELISA recombinante v 3. 0 (FDA 510 k and CE) WHO Consultation Meeting 27 -28 January 2009

Kits ü New Chagatest ELISA recombinante v. 4. 0 (approved in LA/CE market) ü Chagatest ELISA recombinante for dried blood spot samples (approved in RA) ü Rapid test (in development) ü Colorimetric PCR (in development) ü Quantitative PCR (to start development this year) WHO Consultation Meeting 27 -28 January 2009

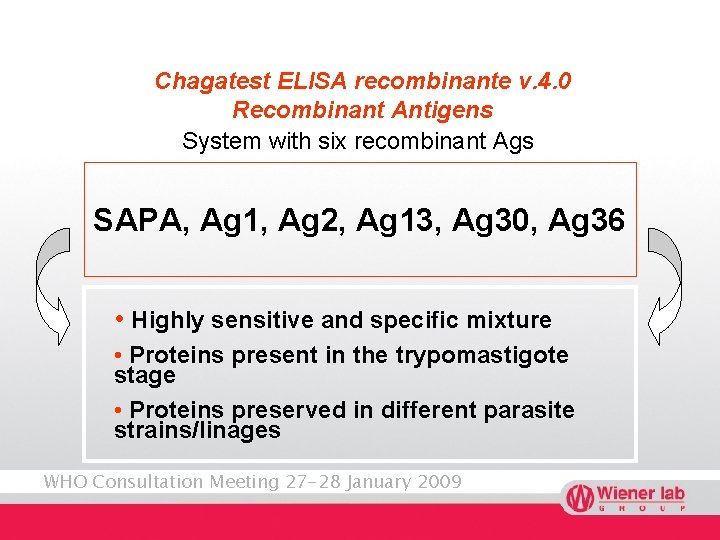
Chagatest ELISA recombinante v. 4. 0 Recombinant Antigens System with six recombinant Ags SAPA, Ag 1, Ag 2, Ag 13, Ag 30, Ag 36 • Highly sensitive and specific mixture • Proteins present in the trypomastigote stage • Proteins preserved in different parasite strains/linages WHO Consultation Meeting 27 -28 January 2009

Chagatest ELISA recombinante v. 4. 0 Recombinant Antigens Ag 1 Ag 2 Ag 30 Ag 13 Ag 36 Ag 2 Ag 13 SAPA Ag 13 Ag 36 SAPA Chronic Congenital Acute WHO Consultation Meeting 27 -28 January 2009

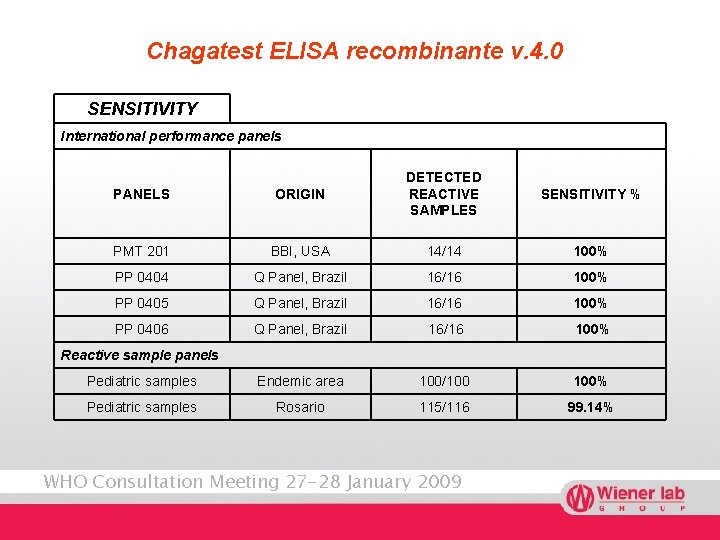
Chagatest ELISA recombinante v. 4. 0 SENSITIVITY International performance panels PANELS ORIGIN DETECTED REACTIVE SAMPLES PMT 201 BBI, USA 14/14 100% PP 0404 Q Panel, Brazil 16/16 100% PP 0405 Q Panel, Brazil 16/16 100% PP 0406 Q Panel, Brazil 16/16 100% Pediatric samples Endemic area 100/100 100% Pediatric samples Rosario 115/116 99. 14% SENSITIVITY % Reactive sample panels WHO Consultation Meeting 27 -28 January 2009

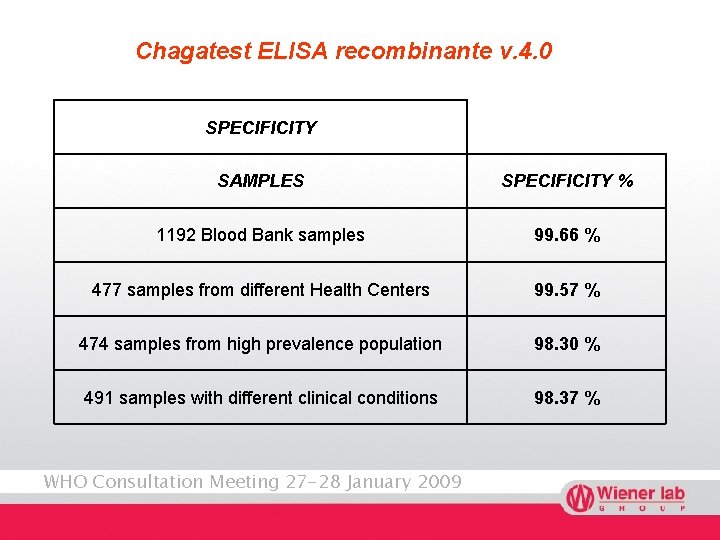
Chagatest ELISA recombinante v. 4. 0 SPECIFICITY SAMPLES SPECIFICITY % 1192 Blood Bank samples 99. 66 % 477 samples from different Health Centers 99. 57 % 474 samples from high prevalence population 98. 30 % 491 samples with different clinical conditions 98. 37 % WHO Consultation Meeting 27 -28 January 2009

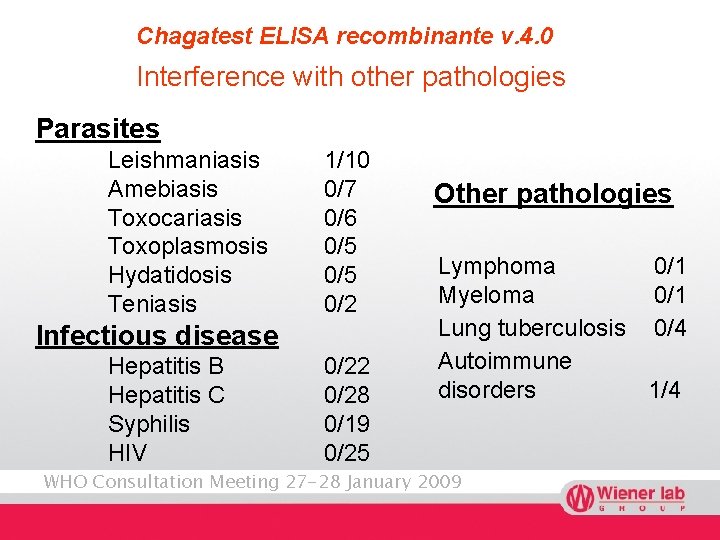
Chagatest ELISA recombinante v. 4. 0 Interference with other pathologies Parasites Leishmaniasis Amebiasis Toxocariasis Toxoplasmosis Hydatidosis Teniasis 1/10 0/7 0/6 0/5 0/2 Infectious disease Hepatitis B Hepatitis C Syphilis HIV 0/22 0/28 0/19 0/25 Other pathologies Lymphoma 0/1 Myeloma 0/1 Lung tuberculosis 0/4 Autoimmune disorders 1/4 WHO Consultation Meeting 27 -28 January 2009

Recombinant antigens Advantages in serological diagnosis ü Standardized system uses a perfectly defined antigen composition. ü Antigens expressed in the infected trypomastigote stage of the parasite. ü Highly preserved antigens in different strains of the parasite. ü SAPA antigen, acute and congenital infection marker. WHO Consultation Meeting 27 -28 January 2009

How do we ensure Standardization? - At production level Recombinant antigens ü Recombinant antigens well characterized ü Perfectly defined antigen mix Parasitic Lysate ü Characterized lysate by WB ü Parasite culture under strict growth conditions. Well defined WCB & MCB WHO Consultation Meeting 27 -28 January 2009

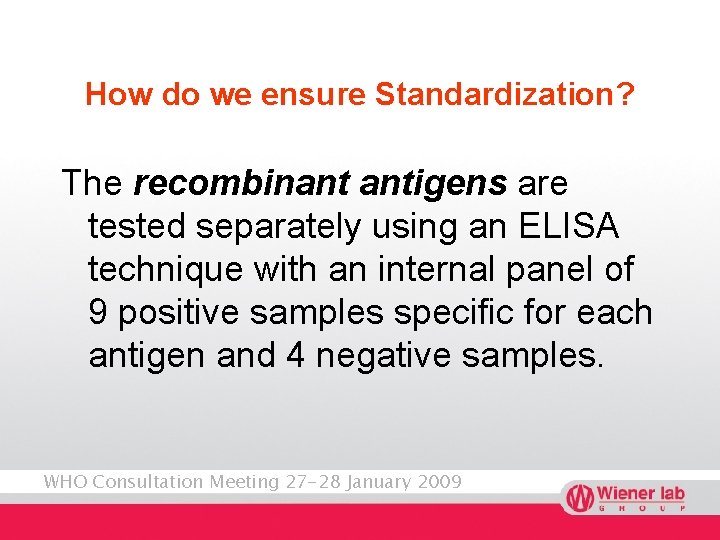
How do we ensure Standardization? The recombinant antigens are tested separately using an ELISA technique with an internal panel of 9 positive samples specific for each antigen and 4 negative samples. WHO Consultation Meeting 27 -28 January 2009

How do we ensure Standardization? ELISAs Calibration 10 weak positive samples (IP between 1. 0 and 2. 0) 10 medium positive samples (IP between 2. 0 and 4. 0) 10 strong positive samples (IP < 4. 0) Note: samples diluted in negative or bovine serum may be used 20 negative samples WHO Consultation Meeting 27 -28 January 2009

How do we ensure Standardization? ELISAs Calibration A titer verifying the IP coefficient within a range of 0. 9 – 1. 2 must be selected. In addition, all negative samples must yield negative results. WHO Consultation Meeting 27 -28 January 2009

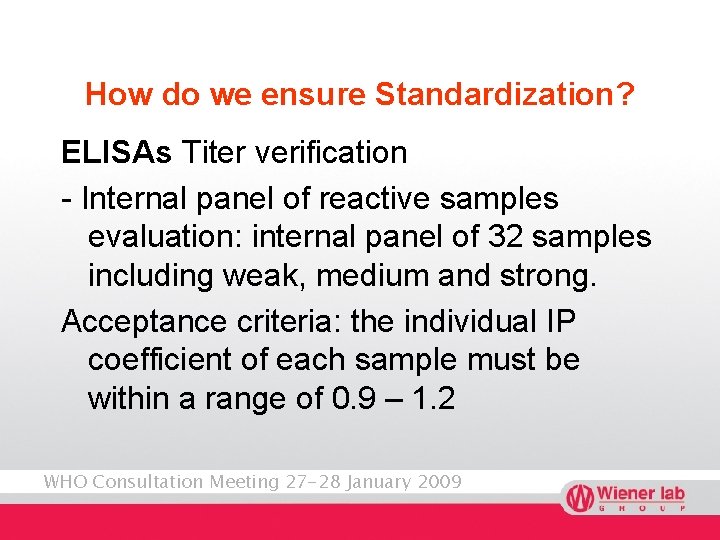
How do we ensure Standardization? ELISAs Titer verification - Internal panel of reactive samples evaluation: internal panel of 32 samples including weak, medium and strong. Acceptance criteria: the individual IP coefficient of each sample must be within a range of 0. 9 – 1. 2 WHO Consultation Meeting 27 -28 January 2009

How do we ensure Standardization? ELISAs Titer verification - Specificity: 200 sera / fresh plasmas Acceptance criteria: > 99%. If < 99%, the conjugate is diluted and retested. WHO Consultation Meeting 27 -28 January 2009

How do we ensure Standardization? ELISAs Final test - Internal panel: 2 positive control sera, 3 negative control sera, 6 weak positive, 10 medium and 10 strong samples. - Commercial panels: Chagas performance panel (QPanel, Brazil); BBI Panel WHO Consultation Meeting 27 -28 January 2009

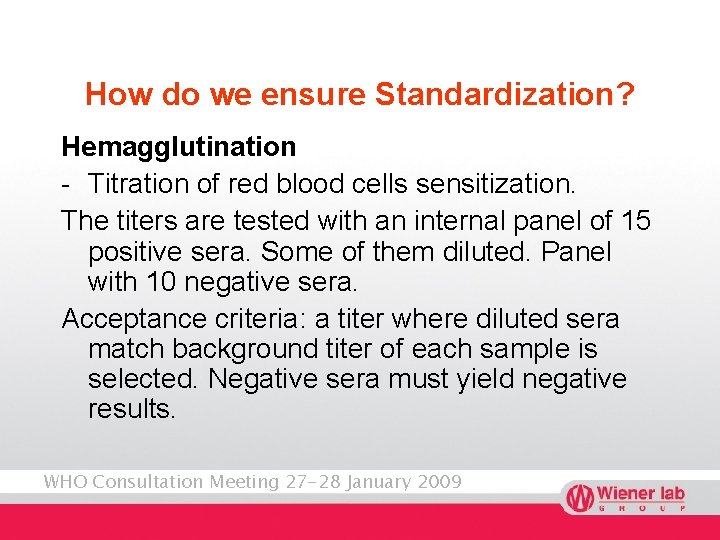
How do we ensure Standardization? Hemagglutination - Titration of red blood cells sensitization. The titers are tested with an internal panel of 15 positive sera. Some of them diluted. Panel with 10 negative sera. Acceptance criteria: a titer where diluted sera match background titer of each sample is selected. Negative sera must yield negative results. WHO Consultation Meeting 27 -28 January 2009

How do we ensure Standardization? Hemagglutination Lot preparation - Internal panel of 20 positive sera - 100 negative sera WHO Consultation Meeting 27 -28 January 2009

How do we ensure Standardization? Hemagglutination Final verification - Chagas performance panel (QPanel) - BBI panel WHO Consultation Meeting 27 -28 January 2009

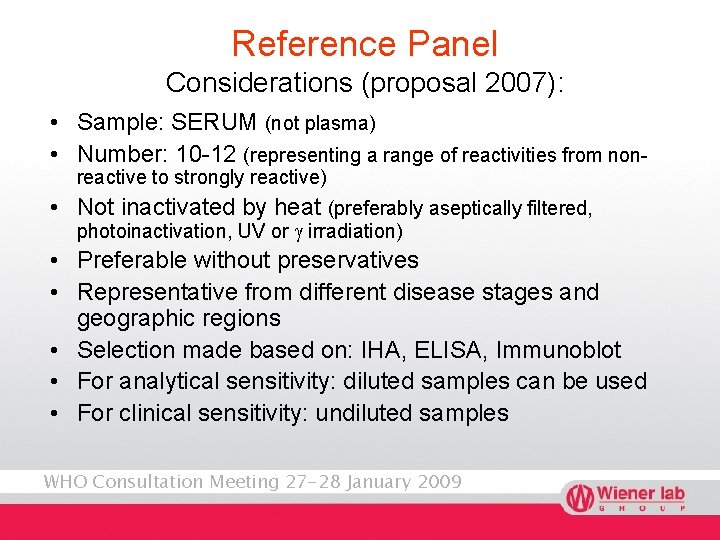
Reference Panel Considerations (proposal 2007): • Sample: SERUM (not plasma) • Number: 10 -12 (representing a range of reactivities from nonreactive to strongly reactive) • Not inactivated by heat (preferably aseptically filtered, photoinactivation, UV or g irradiation) • Preferable without preservatives • Representative from different disease stages and geographic regions • Selection made based on: IHA, ELISA, Immunoblot • For analytical sensitivity: diluted samples can be used • For clinical sensitivity: undiluted samples WHO Consultation Meeting 27 -28 January 2009

International Biological Reference Preparation for Chagas (2009) • A known reactivity standard is required to yield consistency lot to lot • To have a primary Standard of 2 reactive sera, as suggested, seems a good alternative. • This will allow to determine the analytical sensitivity for each lot, as being used for other international standards. WHO Consultation Meeting 27 -28 January 2009
 Quality control assay
Quality control assay Tripomastigotas de trypanosoma cruzi
Tripomastigotas de trypanosoma cruzi Sintomas fase aguda doença de chagas
Sintomas fase aguda doença de chagas Disenteria amebiana
Disenteria amebiana Take-home message examples
Take-home message examples Oncocercose
Oncocercose Instituto evandro chagas
Instituto evandro chagas Quality assurance vs quality control
Quality assurance vs quality control Project quality management pmp
Project quality management pmp What are quality standards in project management
What are quality standards in project management Basic concepts of quality assurance
Basic concepts of quality assurance Gm strategy based diagnostics
Gm strategy based diagnostics Duct diagnostics
Duct diagnostics Customer journey diagnostics
Customer journey diagnostics Win loader
Win loader Standard diagnostics inc
Standard diagnostics inc Erd commander 2010
Erd commander 2010 Laser beam diagnostics
Laser beam diagnostics Ergothérapeute questions d'entretien d'embauche
Ergothérapeute questions d'entretien d'embauche Birafs
Birafs Cole diagnostics jobs
Cole diagnostics jobs Casewise diagnostics
Casewise diagnostics Cefapodoxime
Cefapodoxime Ramsay scale
Ramsay scale Strongstep diagnostics
Strongstep diagnostics