Quality Assurance Test of Delivered Dose Uniformity of













- Slides: 30

Quality Assurance Test of Delivered Dose Uniformity of Multi-dose Spray and Inhalation Drug Products Drs. Yi Tsong 1, Xiaoyu (Cassie) Dong*1, Meiyu Shen 1& Richard T. Lostritto 2 1: Office of Biostatistics/Office of Translational Sciences, CDER, FDA 2: Office of Pharmaceutical Quality, CDER, FDA 2015 MBSW 1

Disclaimer • This article reflects the views of the authors and should not be construed to represent FDA’s views or policies. 2015 MBSW 2

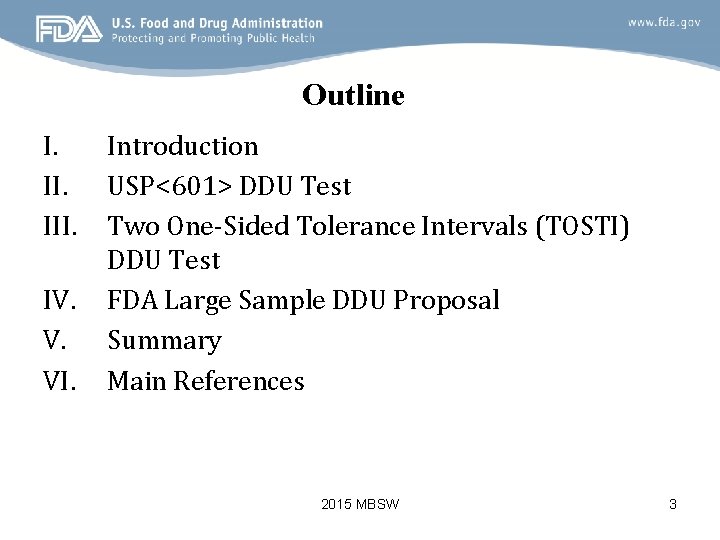
Outline I. III. IV. V. VI. Introduction USP<601> DDU Test Two One-Sided Tolerance Intervals (TOSTI) DDU Test FDA Large Sample DDU Proposal Summary Main References 2015 MBSW 3

I. Introduction 2015 MBSW 4

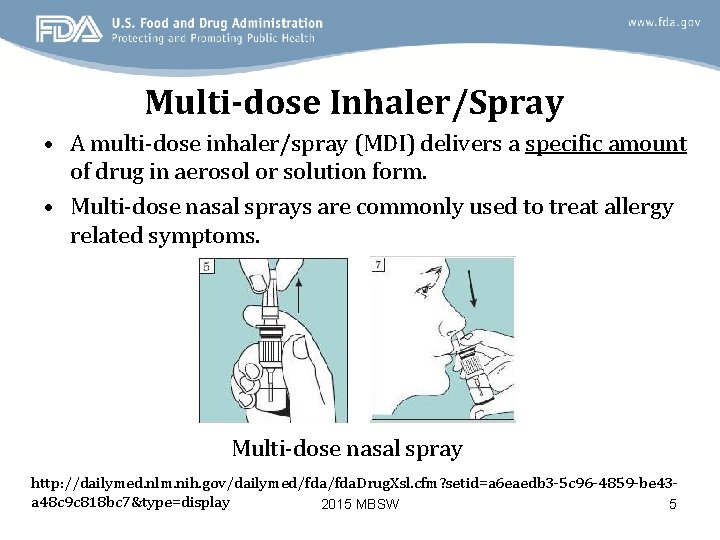
Multi-dose Inhaler/Spray • A multi-dose inhaler/spray (MDI) delivers a specific amount of drug in aerosol or solution form. • Multi-dose nasal sprays are commonly used to treat allergy related symptoms. Multi-dose nasal spray http: //dailymed. nlm. nih. gov/dailymed/fda. Drug. Xsl. cfm? setid=a 6 eaedb 3 -5 c 96 -4859 -be 43 a 48 c 9 c 818 bc 7&type=display 2015 MBSW 5

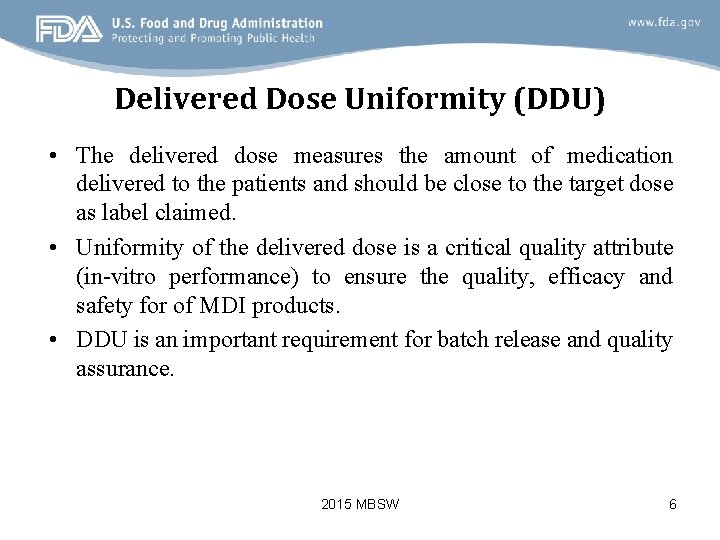
Delivered Dose Uniformity (DDU) • The delivered dose measures the amount of medication delivered to the patients and should be close to the target dose as label claimed. • Uniformity of the delivered dose is a critical quality attribute (in-vitro performance) to ensure the quality, efficacy and safety for of MDI products. • DDU is an important requirement for batch release and quality assurance. 2015 MBSW 6

II. <USP> 601 DDU Test 2015 MBSW 7

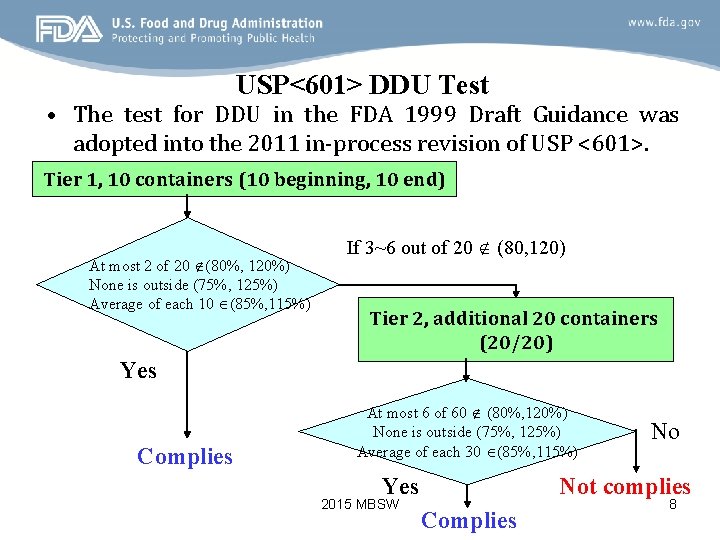
USP<601> DDU Test • The test for DDU in the FDA 1999 Draft Guidance was adopted into the 2011 in-process revision of USP <601>. Tier 1, 10 containers (10 beginning, 10 end) At most 2 of 20 (80%, 120%) None is outside (75%, 125%) Average of each 10 (85%, 115%) If 3~6 out of 20 (80, 120) Tier 2, additional 20 containers (20/20) Yes Complies At most 6 of 60 (80%, 120%) None is outside (75%, 125%) Average of each 30 (85%, 115%) Yes 2015 MBSW No Not complies Complies 8

USP<601> DDU Test • A counting method: converts a continues variable (DD) into a binary variable (outside/inside): Sampling by Variable vs. Sampling by Attribute • Only applicable to the samples, not for inference on the entire batch. – No clear definition of the product quality; – Not a statistically based sampling plan. – The sample is OK ≠ The batch is OK. • Zero Tolerance Outside (75%, 125%): – To detect extreme data. – It is no effect for small sample; – Not reward large samples; 2015 MBSW 9

USP<601> DDU Test Is the USP DDU test a batch release test? Can the USP test make inference on the entire batch? No. Only applicable to the samples, not for inference on the entire batch. Is the USP test a stringent test for batch release? No. Whenever tested, the product needs to meet the USP acceptance criteria. Thus, a batch release test is usually more stringent than the USP test. • Needs a statistically sound approach for batch release: the tolerance interval approach controls the coverage within a specific interval (say 80 – 120%LC). 2015 MBSW 10

III. Two One-sided Tolerance Intervals DDU Test 2015 MBSW 11

Two One-sided Tolerance Intervals Procedure (TOSTI) • In 2003, IPAC-RS (International Pharmaceutical Aerosol Consortium on Regulation and Science) proposed a two-sided tolerance interval approach as an improvement for the control of DDU of orally inhaled and nasal drug products. • Coverage as the quality requirement; • In 2005, FDA proposed a two one-sided tolerance intervals (TOSTI) procedure to test if the batch complies and presented this procedure to the Advisory Committee of Pharmaceutical Science, in October 2005. • Coverage as the quality requirement; 2015 MBSW 12

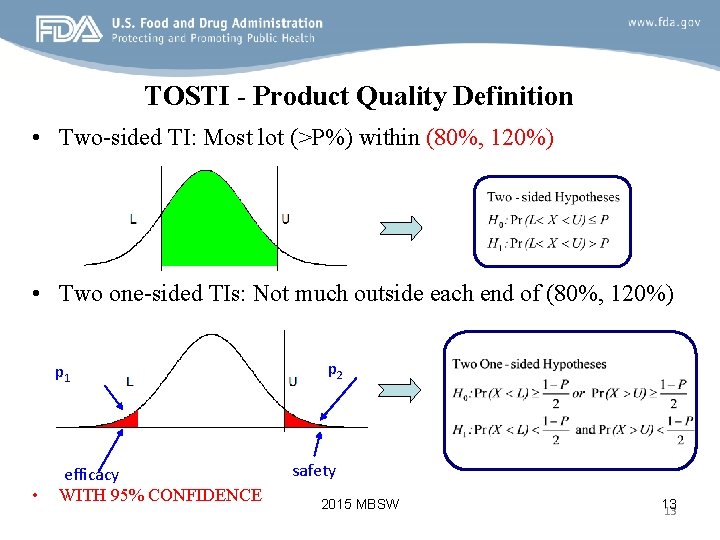
TOSTI - Product Quality Definition • Two-sided TI: Most lot (>P%) within (80%, 120%) • Two one-sided TIs: Not much outside each end of (80%, 120%) p 1 • efficacy WITH 95% CONFIDENCE p 2 safety 2015 MBSW 13 13

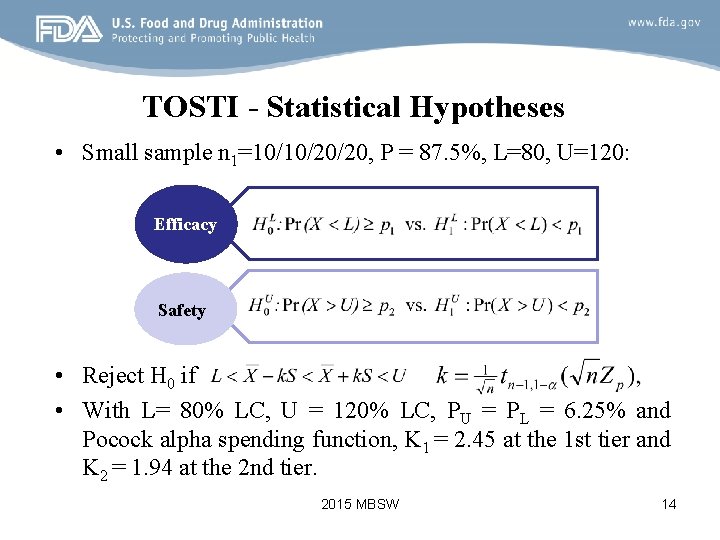
TOSTI - Statistical Hypotheses • Small sample n 1=10/10/20/20, P = 87. 5%, L=80, U=120: Efficacy Safety • Reject H 0 if • With L= 80% LC, U = 120% LC, PU = PL = 6. 25% and Pocock alpha spending function, K 1 = 2. 45 at the 1 st tier and K 2 = 1. 94 at the 2 nd tier. 2015 MBSW 14

TOSTI – Test Flow Chart Tier 1, 10 containers (10 beginning, 10 end) Tier 2, additional 20 containers (20 beginning, 20 end) Average of each 10 (85%, 115%) Yes Complies Average of each 30 (85%, 115%) 2015 MBSW Yes Complies No Not complies 15

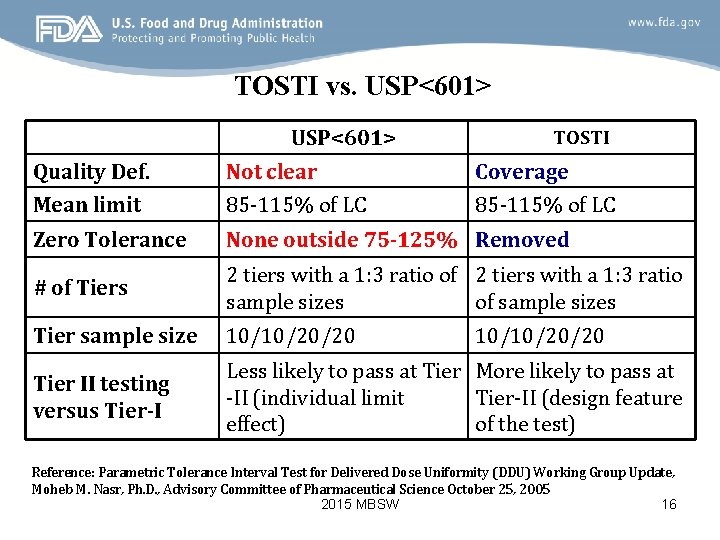
TOSTI vs. USP<601> TOSTI Quality Def. Not clear Coverage Mean limit 85 -115% of LC Zero Tolerance None outside 75 -125% Removed # of Tiers 2 tiers with a 1: 3 ratio of 2 tiers with a 1: 3 ratio sample sizes of sample sizes Tier sample size 10/10/20/20 Tier II testing versus Tier-I Less likely to pass at Tier More likely to pass at -II (individual limit Tier-II (design feature effect) of the test) 10/10/20/20 Reference: Parametric Tolerance Interval Test for Delivered Dose Uniformity (DDU) Working Group Update, Moheb M. Nasr, Ph. D. , Advisory Committee of Pharmaceutical Science October 25, 2005 2015 MBSW 16

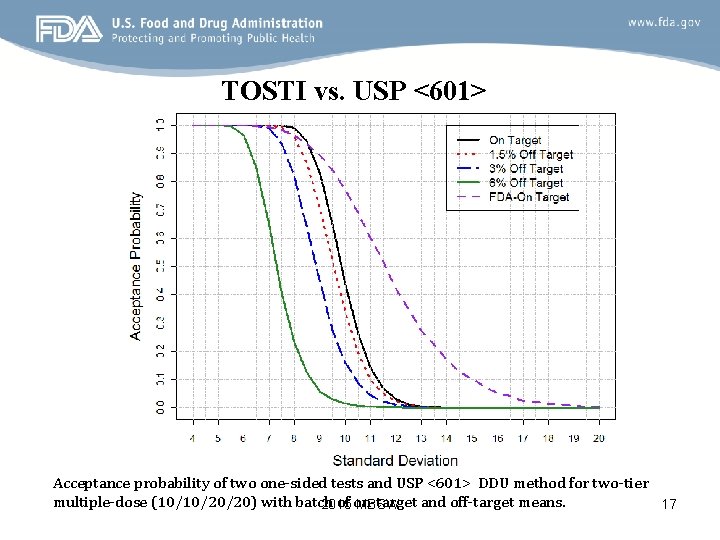
TOSTI vs. USP <601> Acceptance probability of two one-sided tests and USP <601> DDU method for two-tier multiple-dose (10/10/20/20) with batch of on-target and off-target means. 2015 MBSW 17

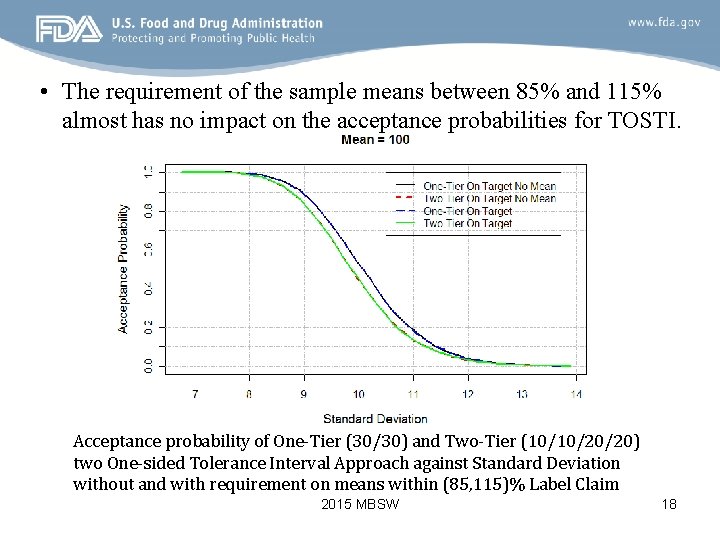
• The requirement of the sample means between 85% and 115% almost has no impact on the acceptance probabilities for TOSTI. Acceptance probability of One-Tier (30/30) and Two-Tier (10/10/20/20) two One-sided Tolerance Interval Approach against Standard Deviation without and with requirement on means within (85, 115)% Label Claim 2015 MBSW 18

IV. FDA Large Sample DDU Proposal 2015 MBSW 19

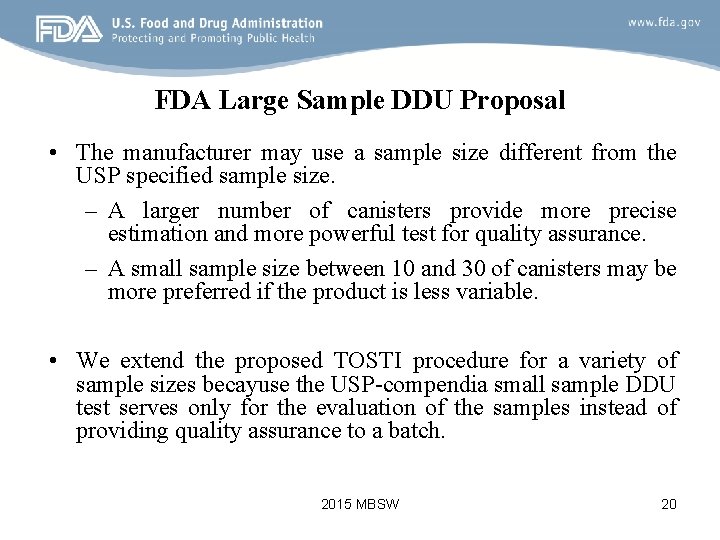
FDA Large Sample DDU Proposal • The manufacturer may use a sample size different from the USP specified sample size. – A larger number of canisters provide more precise estimation and more powerful test for quality assurance. – A small sample size between 10 and 30 of canisters may be more preferred if the product is less variable. • We extend the proposed TOSTI procedure for a variety of sample sizes becayuse the USP-compendia small sample DDU test serves only for the evaluation of the samples instead of providing quality assurance to a batch. 2015 MBSW 20

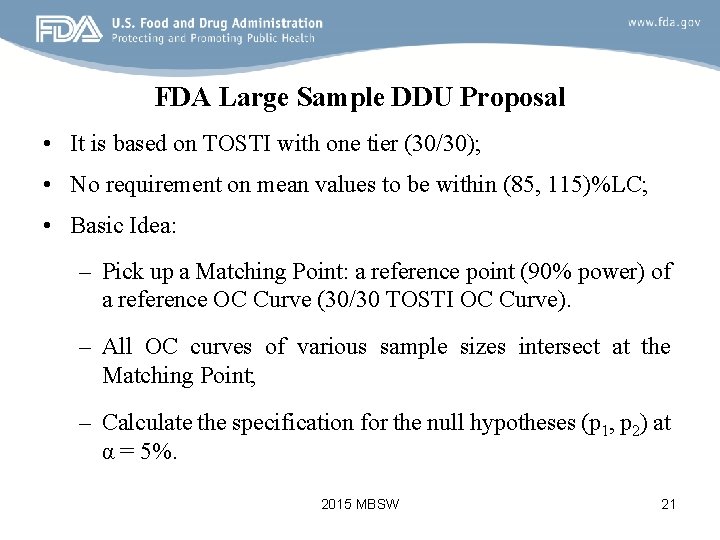
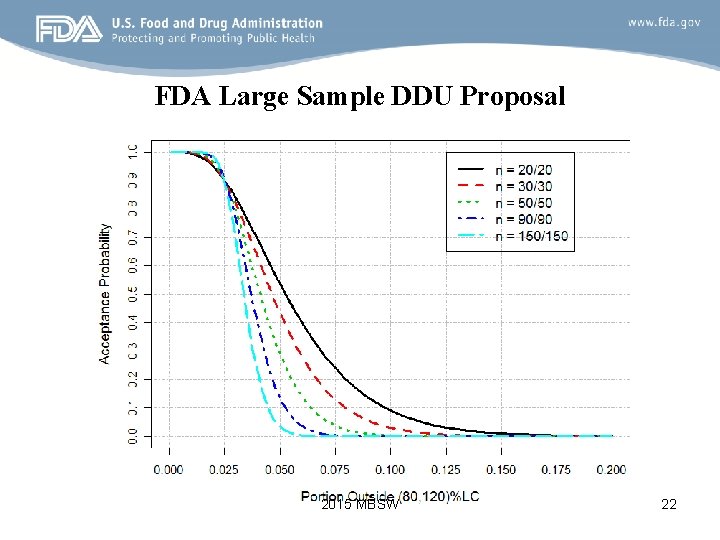
FDA Large Sample DDU Proposal • It is based on TOSTI with one tier (30/30); • No requirement on mean values to be within (85, 115)%LC; • Basic Idea: – Pick up a Matching Point: a reference point (90% power) of a reference OC Curve (30/30 TOSTI OC Curve). – All OC curves of various sample sizes intersect at the Matching Point; – Calculate the specification for the null hypotheses (p 1, p 2) at α = 5%. 2015 MBSW 21

FDA Large Sample DDU Proposal 2015 MBSW 22

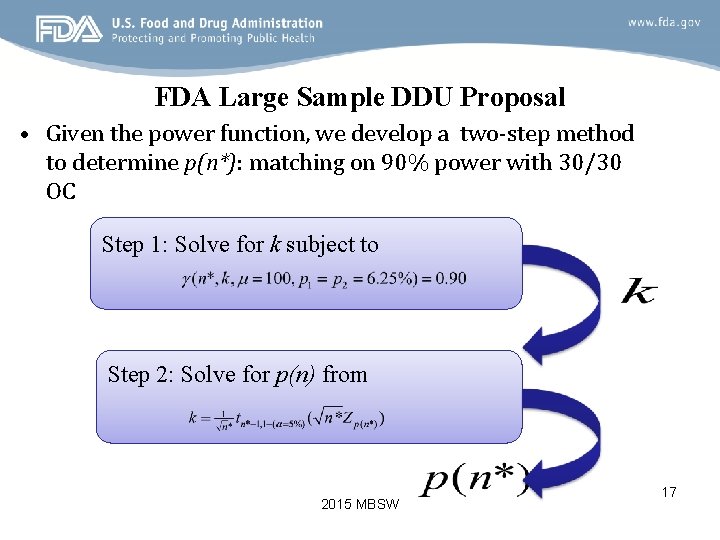
FDA Large Sample DDU Proposal • Given the power function, we develop a two-step method to determine p(n*): matching on 90% power with 30/30 OC Step 1: Solve for k subject to Step 2: Solve for p(n) from 2015 MBSW 17

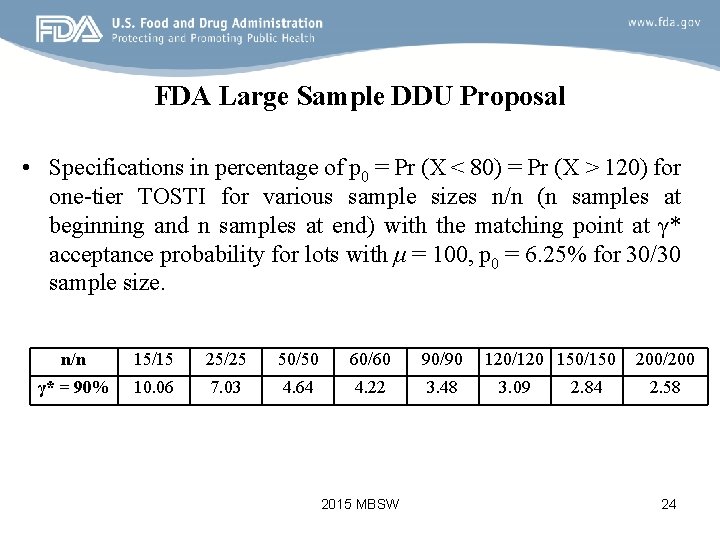
FDA Large Sample DDU Proposal • Specifications in percentage of p 0 = Pr (X < 80) = Pr (X > 120) for one-tier TOSTI for various sample sizes n/n (n samples at beginning and n samples at end) with the matching point at γ* acceptance probability for lots with μ = 100, p 0 = 6. 25% for 30/30 sample size. n/n 15/15 25/25 50/50 60/60 90/90 γ* = 90% 10. 06 7. 03 4. 64 4. 22 3. 48 2015 MBSW 120/120 150/150 3. 09 2. 84 200/200 2. 58 24

V. Summary 2015 MBSW 25

Summary • TOSTI is a batch release test which controls the quality by the coverage within (80%, 120%) of the label claim; • Furthermore, the TOSTI approach accepts a batch only if both portions of units being under-delivered (e. g. <80% efficacy concern) and over-delivered (e. g. > 120% safety concern) are controlled. • It can be adjusted for a two-tier group sequential sampling acceptance plan: – Additional acceptance probability at the 2 nd tier; – More discriminating power between lots with on-target mean and offtarget mean. 2015 MBSW 26

Summary • TOSTI approach can be easily illustrated as a procedure with two one-sided tests or with a two one-sided tolerance intervals concept with exact solutions. • When using a single-tier sampling plan, the TOSTI procedure can also be extended to any sample size. The extension was made by protecting the acceptance rate for lots considered to be high quality in DDU. 2015 MBSW 27

Main References • • FDA Draft Guidance (1998) “FDA/CDER. Guidance for Industry “Nasal Spray and Inhalation Solution, Suspension, and Spray Drug Products--Chemistry, Manufacturing, and Controls Documentation”. Draft: May 1999”. http: //www. fda. gov/downloads/drugs/guidancecomplianceregulatoryinform ation/guidances/ucm 070575. pdf Tsong, Y. , Dong, X. , Shen, M. , Lostritto R. T. (2015). “Quality assurance test of delivered dose uniformity of multiple-dose inhaler and dry powder inhaler drug products”. Journal of Biopharmaceutical Statistics. 25(2): 328 -38. Tsong Y, Shen M, Lostritto RT, Poochikian GK (2008). Parametric two-tier sequential quality assurance test of delivery dose uniformity of multiple-dose inhaler and dry powder inhaler drug products. Journal of Biopharmaceutical Statistics, 18: 5, 976 -984. USP General Chapter <601> Inhalation and Nasal Drug Products: Aerosols, Sprays, and Powders—Performance Quality Tests” http: //www. pharmacopeia. cn/v 29240/usp 29 nf 24 s 0_c 601_viewall. html 2015 MBSW 28

Main References • • Moheb M. Nasr. (2005) “Parametric Tolerance Interval Test for Delivered Dose Uniformity (DDU) Working Group Update”, Ph. D. , Advisory Committee of Pharmaceutical Science October 25, 2005 Bo Olsson (2003), “A Parametric Tolerance Interval Test for Improved Control of Delivered Dose Uniformity of Orally Inhaled and Nasal Drug Products. ” IPACRS Presentaion, Rockville, MD 2015 MBSW 29

Thank you! 2015 MBSW 30
 Delivered dose uniformity
Delivered dose uniformity Perform quality assurance
Perform quality assurance Plan quality management pmp
Plan quality management pmp What are quality standards in project management
What are quality standards in project management Quality assurance cycle in nursing
Quality assurance cycle in nursing Compliance vs quality
Compliance vs quality Basic concept of quality control and quality assurance pdf
Basic concept of quality control and quality assurance pdf Content uniformity usp
Content uniformity usp Water filters for pseudomonas
Water filters for pseudomonas Content uniformity test definition
Content uniformity test definition Isolux diagram for finding luminance on road surface is
Isolux diagram for finding luminance on road surface is Photo response non uniformity
Photo response non uniformity Inspect uniformity of skin color rationale
Inspect uniformity of skin color rationale Uniformity
Uniformity Uniformity
Uniformity Psfm maintainance
Psfm maintainance Tablets quality control tests
Tablets quality control tests Esrlight
Esrlight Friabilator function
Friabilator function Sqa software development
Sqa software development Software testing and quality assurance theory and practice
Software testing and quality assurance theory and practice Defect arrival pattern
Defect arrival pattern Iso 9001 software quality assurance
Iso 9001 software quality assurance Software quality assurance planning
Software quality assurance planning Quality control in blood banking pdf
Quality control in blood banking pdf Software quality assurance challenges
Software quality assurance challenges Control chapter 10
Control chapter 10 Iuqb
Iuqb Contoh quality assurance di rumah sakit
Contoh quality assurance di rumah sakit Pharmacovigilance quality assurance
Pharmacovigilance quality assurance Data entry quality control
Data entry quality control