Qualitative Vs Quantitative Qualitative descriptions or distinctions are













- Slides: 17

Qualitative Vs. Quantitative Qualitative descriptions or distinctions are based on some quality or characteristic rather than on some quantity or measured value.

Qualitative Vs. Quantitative deals with quantities, or information dealing with measureable data, or actual numbers.

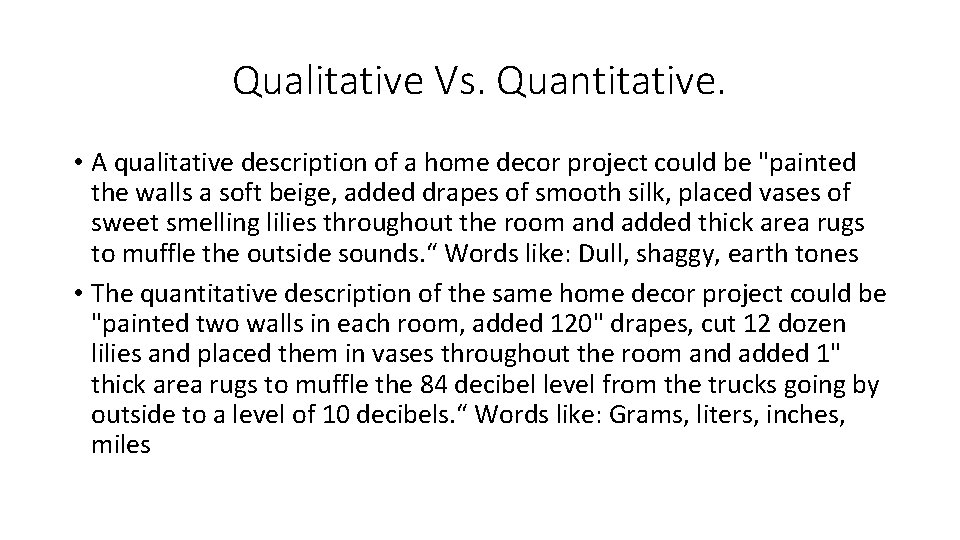
Qualitative Vs. Quantitative. • A qualitative description of a home decor project could be "painted the walls a soft beige, added drapes of smooth silk, placed vases of sweet smelling lilies throughout the room and added thick area rugs to muffle the outside sounds. “ Words like: Dull, shaggy, earth tones • The quantitative description of the same home decor project could be "painted two walls in each room, added 120" drapes, cut 12 dozen lilies and placed them in vases throughout the room and added 1" thick area rugs to muffle the 84 decibel level from the trucks going by outside to a level of 10 decibels. “ Words like: Grams, liters, inches, miles

Filtration

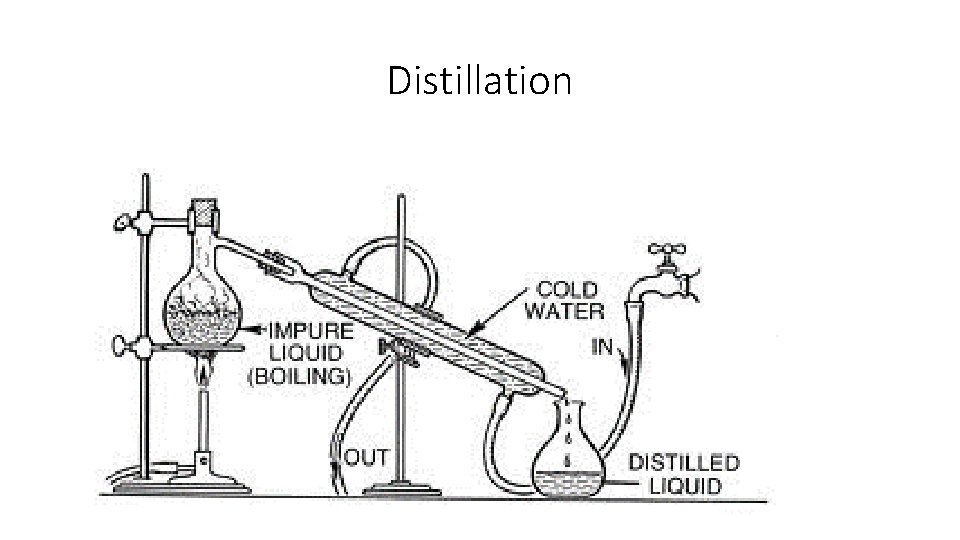
Distillation

Review • States of Matter: • Solids: Have a fixed shape and volume, particles can’t move closer together, and will turn into a liquid if heated (energy added). • Liquid: Have a variable shape and fixed volume, particles can’t move closer together, and will turn into a solid if energy is taken away, or into a gas if energy is added. • Gas: Have a variable shape and volume, particles can move closer together, and will turn into a liquid if energy is taken away.


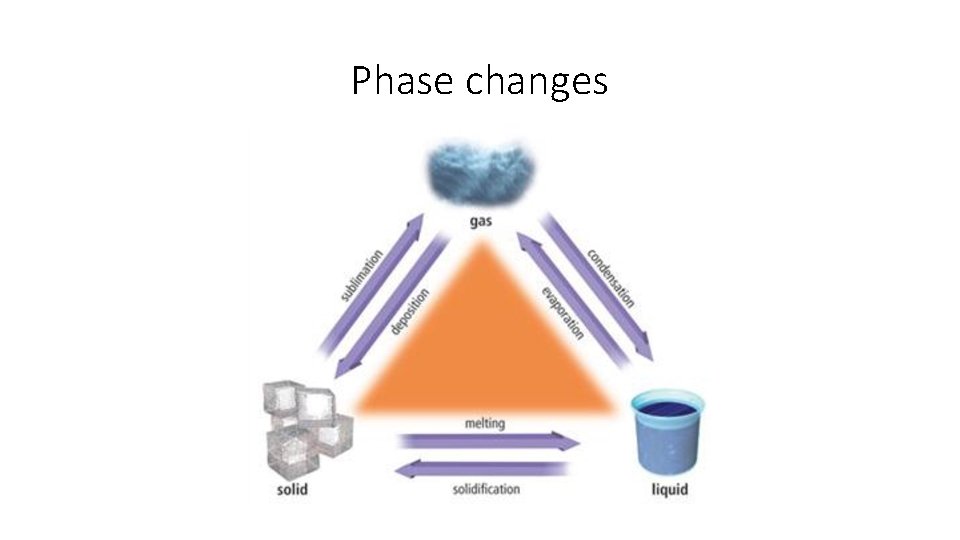
Phase changes

Vocab • Matter: Matter is anything that has mass and takes up space. • (Is light matter? What about heat? Do they have mass? Do they take up space? ) • Element: A substance that cannot be broken down into simpler substances by chemical means. • Compound: A chemical composition of two or more different elements joined together in fixed proportions. • Mixture: A mixture refers to the physical combination of two or more substances on which the identities of each are retained.

Element, Compound, or Mixture?

Physical and Chemical Properties • Two types: Chemical and Physical. • Physical properties do not change the chemical composition! • Physical = Color, Density, Hardness, Boiling/Freezing/Melting point, Smell. • Chemical properties change the chemical composition! • Chemical= Combustion or fire, Changes in p. H, or Chemical reactions!

Physical Properties • Physical has Intensive and Extensive properties. = PIE • Intensive: Do not depend on how much you have! (Color, Luster, Malleability, Conductivity, Melting/Boiling/Freezing Points) • Extensive: Depend on how much you have! (Volume, Mass, Length, Width) • Physical changes do not produce a new substance!

Chemical changes • Produce a new substance! • 1. One or more substances are used up. • 2. One or more new substances are formed. • 3. Energy is either absorbed or released.

Pure Substances • Pure substance: Have a fixed composition but differ from a mixture in the following ways. • EVERY sample of the substance has exactly the same characteristic properties. • EVERY sample of the substance has exactly the same composition. • Pure substances are made up of only ONE element or ONE compound.

Mixtures • Mixture: Is a blend of two or more kinds of matter. Each type of matter retains its own identity and properties. • Two types: Homogeneous and Heterogeneous. • Heterogeneous: Not uniform throughout. • Homogeneous: The same composition throughout. Also known as Solutions.

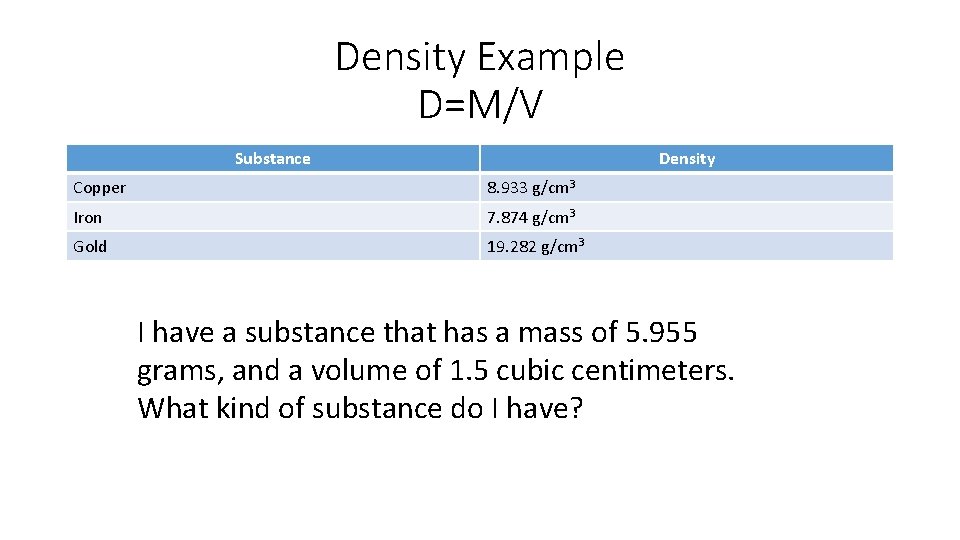
Density • Density = Mass / Volume • Volume = milliliters (ml), or cm 3 • Mass = grams, (g)

Density Example D=M/V Substance Density Copper 8. 933 g/cm 3 Iron 7. 874 g/cm 3 Gold 19. 282 g/cm 3 I have a substance that has a mass of 5. 955 grams, and a volume of 1. 5 cubic centimeters. What kind of substance do I have?