Qualitative Analysis Identifying Ions in Solution Qualitative Analysis







- Slides: 17

Qualitative Analysis Identifying Ions in Solution

Qualitative Analysis involves the use of experimental procedures to determine what elements or ions are present in a substance.

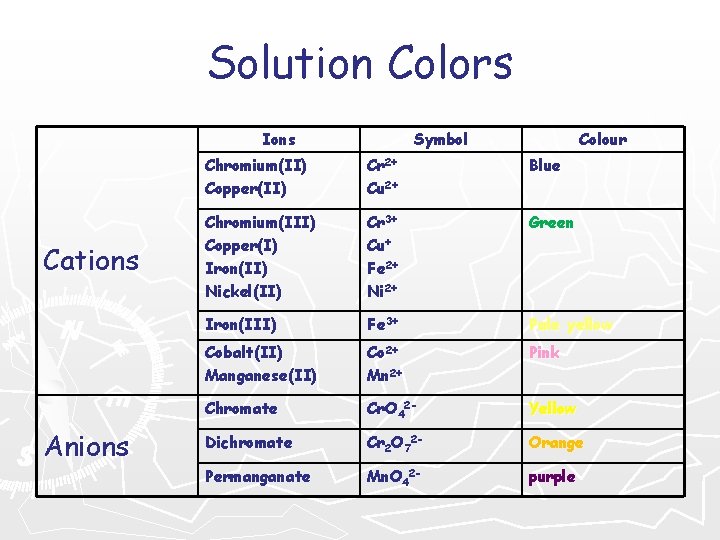
Solution Colors Ions Cations Anions Symbol Colour Chromium(II) Copper(II) Cr 2+ Cu 2+ Blue Chromium(III) Copper(I) Iron(II) Nickel(II) Cr 3+ Cu+ Fe 2+ Ni 2+ Green Iron(III) Fe 3+ Pale yellow Cobalt(II) Manganese(II) Co 2+ Mn 2+ Pink Chromate Cr. O 42 - Yellow Dichromate Cr 2 O 72 - Orange Permanganate Mn. O 42 - purple

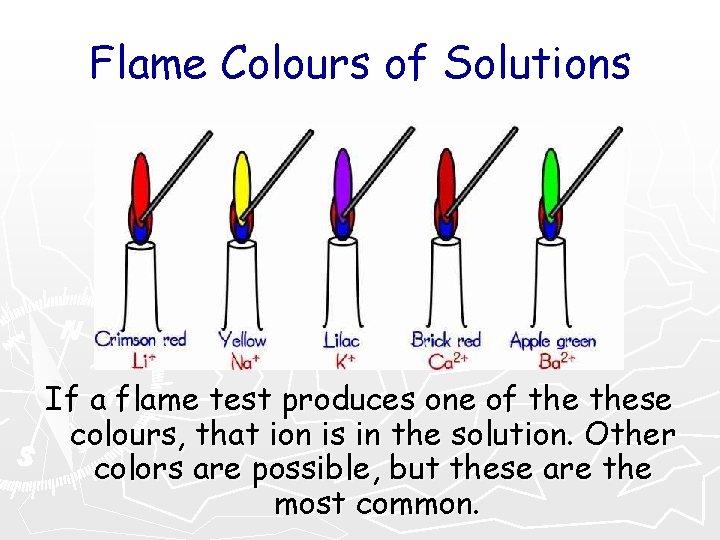
Flame Colours of Solutions If a flame test produces one of these colours, that ion is in the solution. Other colors are possible, but these are the most common.

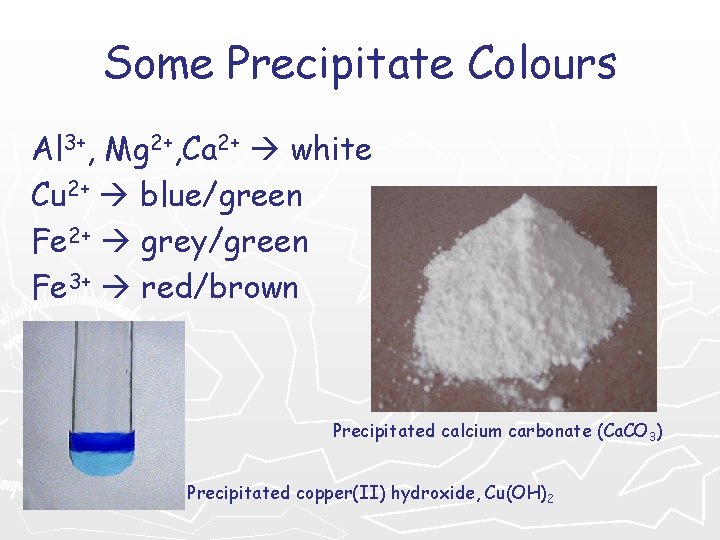
Some Precipitate Colours Al 3+, Mg 2+, Ca 2+ white Cu 2+ blue/green Fe 2+ grey/green Fe 3+ red/brown Precipitated calcium carbonate (Ca. CO 3) Precipitated copper(II) hydroxide, Cu(OH)2

Qualitative analysis using precipitation

For Example: Ag+ and Sr+2 We try to find some anion which could form a precipitate with only one of our two cations at a time. Assume one or both of these cation is in solution If a precipitate is formed, we can then assume that the ion we are looking for is in fact present; if no precipitate forms, the ion is absent. If a precipitate forms, then we filter it off and add another anion to precipitate the second ion. If a precipitate forms, then the second ion is present. If a precipitate does not form, then the second ion is not present in the solution.

Developing a Qualitative Analysis Scheme ► Qualitative Analysis: ► For many ionic substances the decision can be made using the solubility table. ► Looking at the previous example, let’s develop a qualitative analysis scheme.

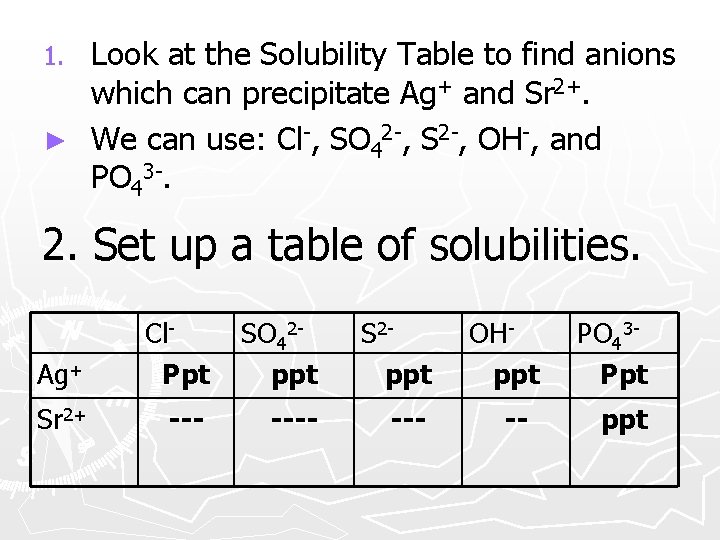
Look at the Solubility Table to find anions which can precipitate Ag+ and Sr 2+. ► We can use: Cl-, SO 42 -, S 2 -, OH-, and PO 43 -. 1. 2. Set up a table of solubilities. Ag+ Cl. Ppt SO 42 ppt Sr 2+ ---- --- OHppt -- PO 43 Ppt ppt

Procedure: 1. Start by adding Cl-, S 2 -, or OH- to try to precipitate Ag+ (we do not use SO 42 - or PO 43 - as they could also ppt Sr 2+). § If a precipitate forms, then there is Ag+ present. Filter off and discard the precipitate. Keep the left over solution for the next part. 2. Add SO 42 - or PO 43 - to try to precipitate Sr 2+. ► If a precipitate forms, then there is Sr 2+ present. ►

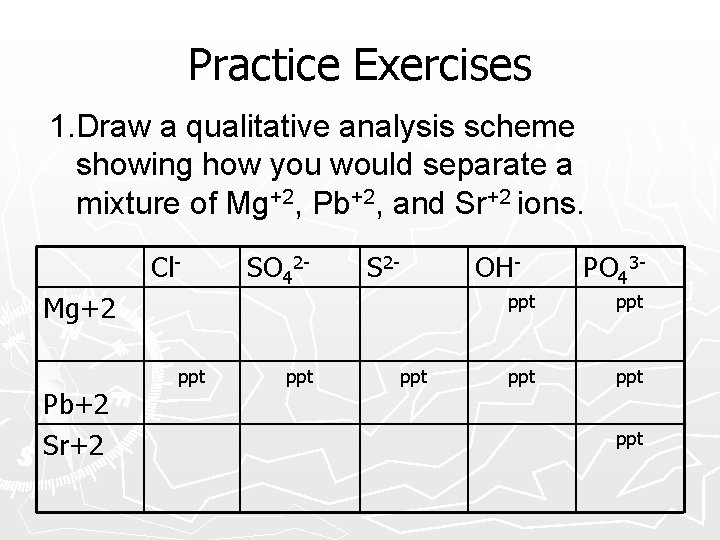
Practice Exercises 1. Draw a qualitative analysis scheme showing how you would separate a mixture of Mg+2, Pb+4, and Sr+2 ions. Cl. Mg+2 Pb+4 Sr+2 SO 42 - S 2 - OH- PO 43 -

Practice Exercises 1. Draw a qualitative analysis scheme showing how you would separate a mixture of Mg+2, Pb+2, and Sr+2 ions. Cl- SO 42 - S 2 - OH- Mg+2 Pb+2 Sr+2 ppt ppt PO 43 - ppt ppt ppt

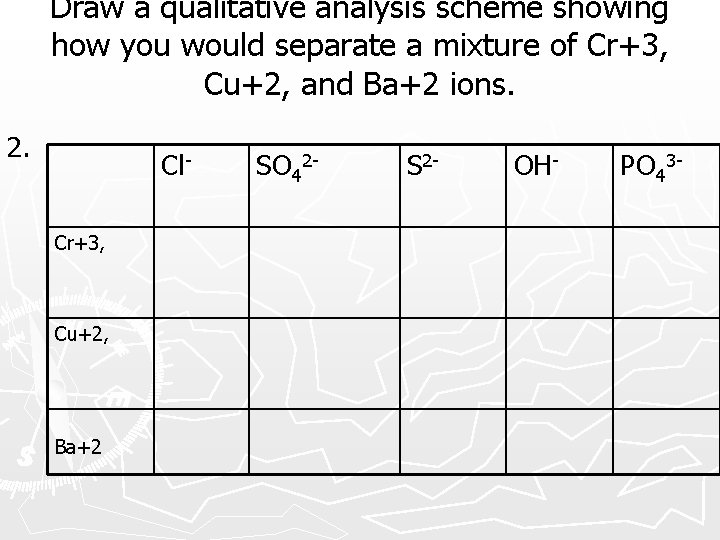
Draw a qualitative analysis scheme showing how you would separate a mixture of Cr+3, Cu+2, and Ba+2 ions. 2. Cl. Cr+3, Cu+2, Ba+2 SO 42 - S 2 - OH- PO 43 -

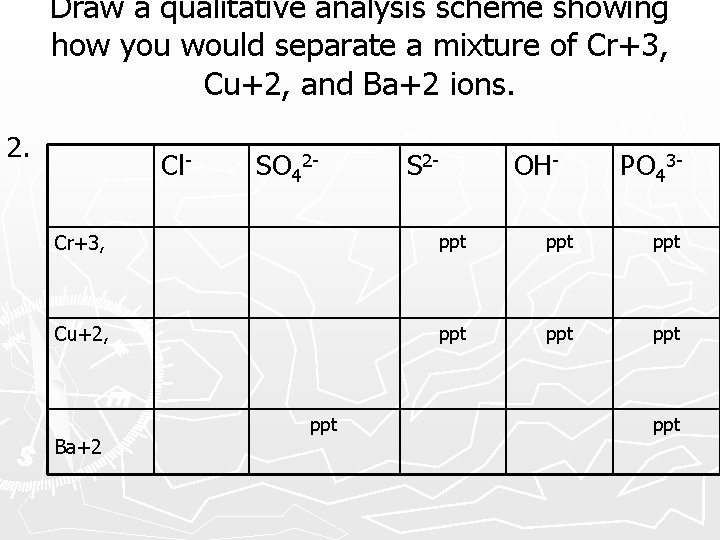
Draw a qualitative analysis scheme showing how you would separate a mixture of Cr+3, Cu+2, and Ba+2 ions. 2. Cl- SO 42 - S 2 - OH- PO 43 - Cr+3, ppt ppt Cu+2, ppt ppt Ba+2 ppt

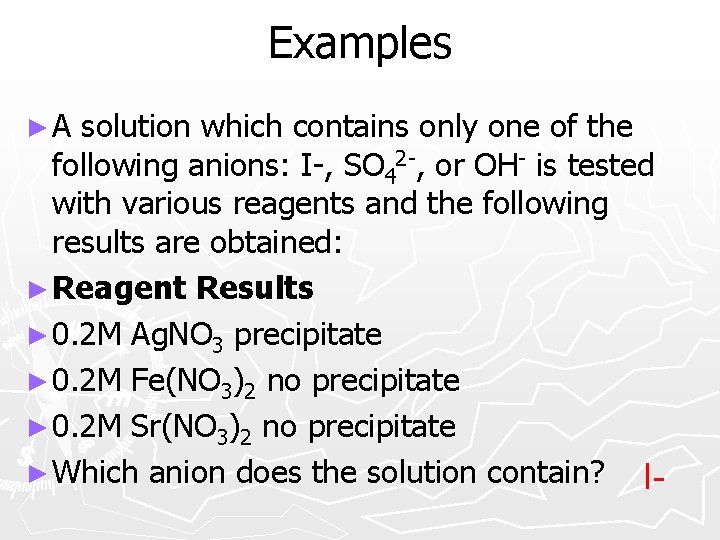
Examples ►A solution which contains only one of the following anions: I-, SO 42 -, or OH- is tested with various reagents and the following results are obtained: ► Reagent Results ► 0. 2 M Ag. NO 3 precipitate ► 0. 2 M Fe(NO 3)2 no precipitate ► 0. 2 M Sr(NO 3)2 no precipitate ► Which anion does the solution contain? I-

Examples cont’d ►A solution which contains only one of the following anions: OH-, SO 42 -, or CO 32► is tested with various reagents and the following results are obtained: ► Reagent Results ► 0. 2 M Ag. NO 3 precipitate ► 0. 2 M Fe(NO 3)2 precipitate ► 0. 2 M Sr(NO 3)2 no precipitate OH ► Which anion does the solution contain? ______________

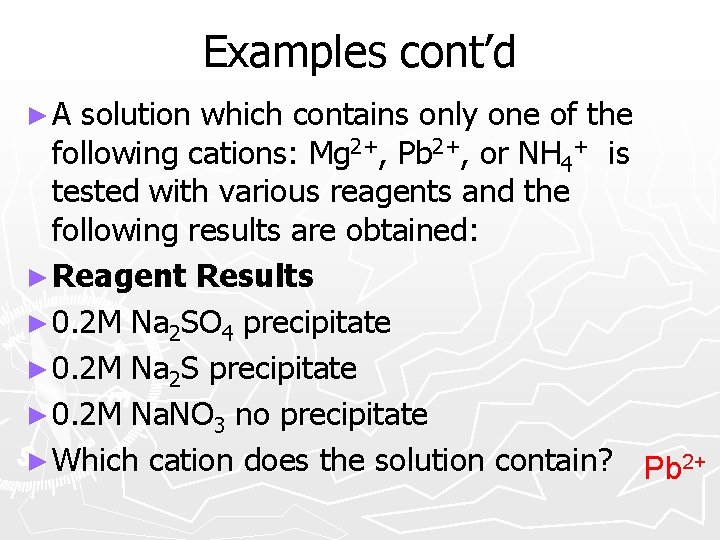
Examples cont’d ►A solution which contains only one of the following cations: Mg 2+, Pb 2+, or NH 4+ is tested with various reagents and the following results are obtained: ► Reagent Results ► 0. 2 M Na 2 SO 4 precipitate ► 0. 2 M Na 2 S precipitate ► 0. 2 M Na. NO 3 no precipitate ► Which cation does the solution contain? Pb 2+