Qualification And Validation In Pharmaceutical Manufacturing KHADIJAH ADEABOLADE

Qualification And Validation In Pharmaceutical Manufacturing KHADIJAH ADE-ABOLADE MPH. FPCPharm. Chief Regulatory Officer/Lead Inspector Drug Evaluation & Research NAFDAC

OBJECTIVES • Discuss the requirements for Qualification and Validation in Pharmaceutical Manufacturing • Appreciate the need for Validation

OUTLINE • • Definitions Validation documentation Qualification Types of validation Approaches Life cycle concept of validation Conclusion

INTRODUCTION • Safety, quality and efficacy are built into the product – cannot be "inspected or tested into a product" • Need for confidence that the product will consistently meet predetermined specifications and attributes

WHAT IS VALIDATION? • “Validation is defined as the collection & evaluation of data, from the process design stage through commercial production, which establishes scientific evidence that a process is capable of consistently delivering quality product. ” • Documenting that a process or system meets its pre-determined specifications and quality attributes

WHAT IS VALIDATION? • EU GMP - it is “Action of proving, in accordance with the principles of Good Manufacturing Practice (GMP), that any procedure, process, equipment, material, activity or system actually leads to expected results. ” • The US FDA defines “Validation as establishing documented evidence which provides high degree of assurance that a specific process will constantly produce a product meeting its pre-determined specification and quality attributes. ”

WHAT IS QUALIFICATION • Performed to establish confidence that process equipment and ancillary systems are capable of consistently operating within established limits and tolerances. • Provides documented evidence that the subject equipment has been installed as per specification (manufacturer’s recommendation) and will attain and maintain critical process parameters repeatedly and reliably. • A subset of validation, typically done as part of a larger validation effort.

Comparison between Qualification and Validation • Validation and Qualification are essentially components of the same concept. • Qualification is normally used for equipment, utilities and systems • Validation is normally used for processes and methods • In this sense, qualification is often a part (the initial stage) of validation but the individual qualification steps alone do not constitute process validation. • Process validation cannot take place without first carrying out qualification

WHY VALIDATION? • Assures Quality • Regulatory Requirement • Reduces Cost • It’s the LAW !
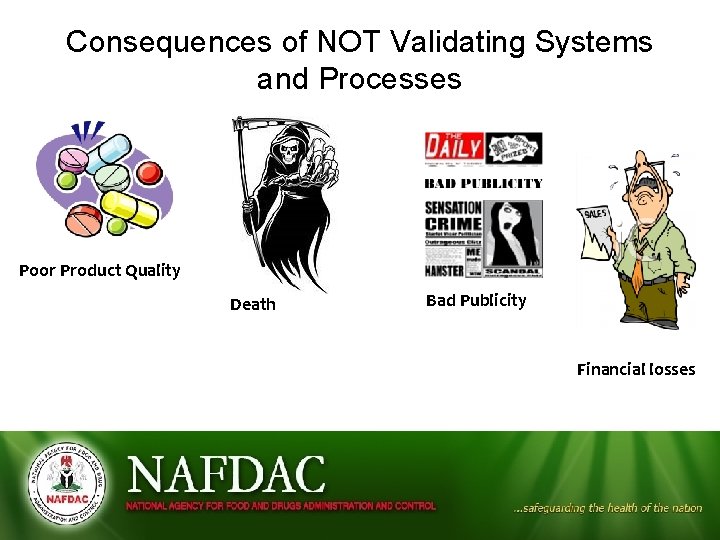
Consequences of NOT Validating Systems and Processes Poor Product Quality Death Bad Publicity Financial losses
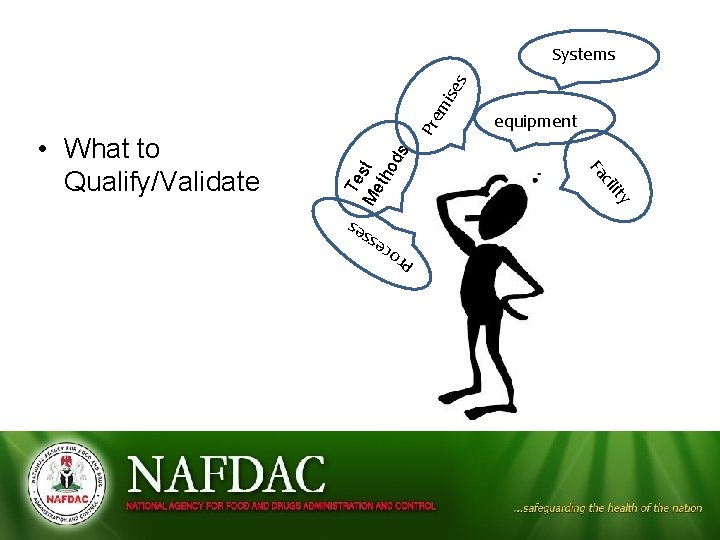
ity Te Me st tho d s equipment cil Fa ce Pr o s sse • What to Qualify/Validate Pre m ise s Systems

Responsibility ü Responsibility for qualification and validation is a multidisciplinary one which includes: ü Heads of Production and QC üHeads of Engineering and Contractors/suppliers üRespective responsibilities clearly defined in the VMP. Pharm. (Mrs) Edosa Ogbeide 12
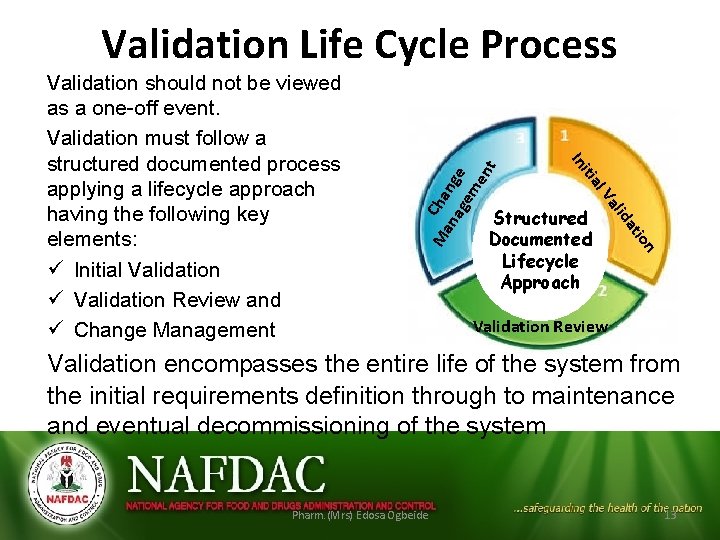
n tio a lid Structured Documented Lifecycle Approach Va Ma Cha na nge ge me n al iti In Validation should not be viewed as a one-off event. Validation must follow a structured documented process applying a lifecycle approach having the following key elements: ü Initial Validation ü Validation Review and ü Change Management t Validation Life Cycle Process Validation Review Validation encompasses the entire life of the system from the initial requirements definition through to maintenance and eventual decommissioning of the system Pharm. (Mrs) Edosa Ogbeide 13
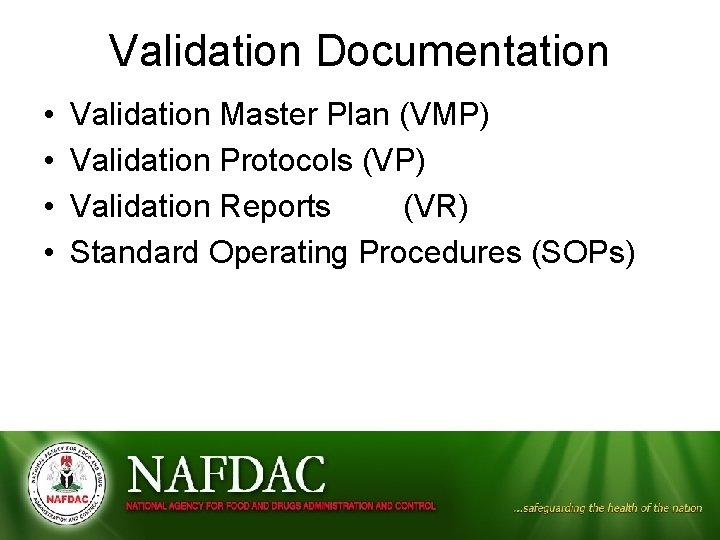
Validation Documentation • • Validation Master Plan (VMP) Validation Protocols (VP) Validation Reports (VR) Standard Operating Procedures (SOPs)

Validation Documentation Validation Master Plan • Contains key elements of the validation program. • Concise, clear, contains at least: qa validation policy qorganizational structure of validation activities qsummary of facilities, systems, equipment and processes validated (and to be validated) qdocumentation format (e. g. protocol and report) qplanning and scheduling qchange control and references to existing documents
Validation Documentation Validation Protocol • A validation protocol is a detailed document relating to a specific part of the validation process. • Outlines tests to be carried out, the acceptance criteria and the information that must be recorded. • Defines the approval process for the validation. • Describes the procedure to be followed for performing validation. • Objectives of the validation/qualification study, the site of the study, the responsible personnel
Validation Documentation Validation Protocol • Description of the equipment to be used (including calibration before and after validation) • SOPs to be followed (e. g. the operation and cleaning of the equipment) and the standards and criteria for the relevant products and processes. • Type of validation and time/frequency should also be stipulated. • Processes and/or parameters to be validated (e. g. mixing times, drying temperatures, drying times, physical characteristics, content uniformity, etc. )

Validation Documentation Validation Report • Record of results obtained during the performance of the validation. • Reflects the final test results and other documents such as instrument calibration certificates. • Basis on which the decision is taken on whether a particular process is judged to be validated. • Includes evaluation, analysis and comparison of results with acceptance criteria by the responsible personnel. • Results should meet acceptance criteria and satisfy the stated objective.
Validation Documentation Validation Report • Refer to the protocol, state details of material, equipment, programs and cycles used, together with details of procedures and test methods. • Include recommendations on the limits and criteria to be applied to all future production batches. • Protocol and the report may be combined into a single set of documents. • The protocol is approved as a form on which the test results are recorded as they become available.

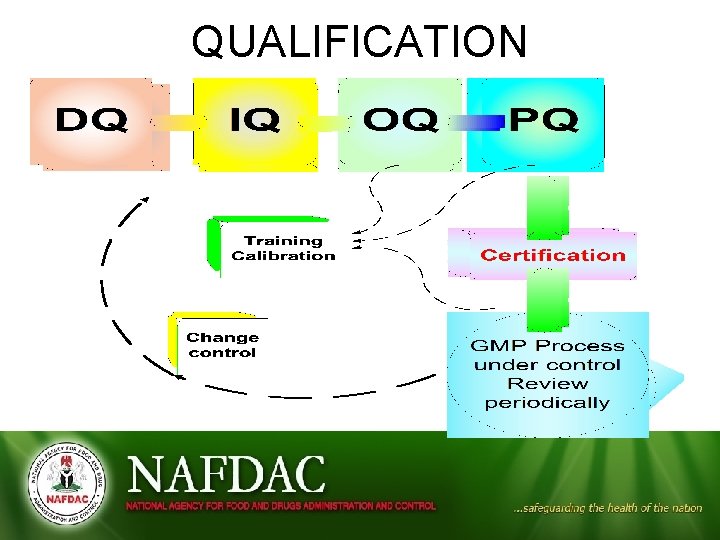
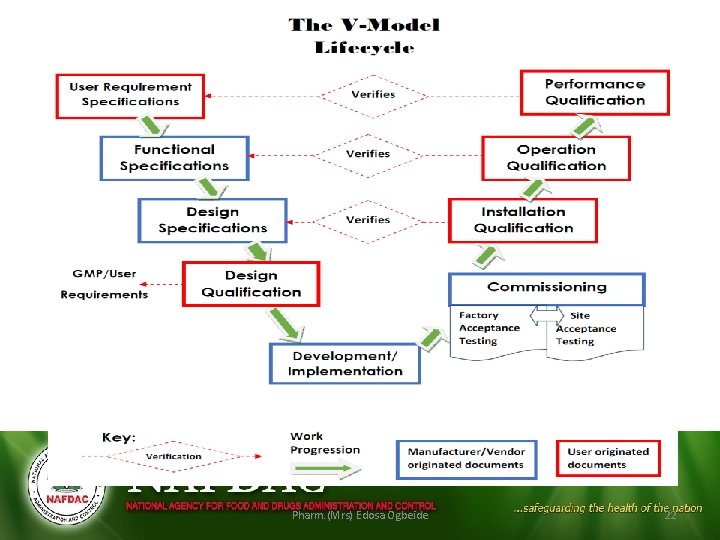
QUALIFICATION Types (Stages) of Qualification • • Design qualification (DQ) Installation qualification (IQ) Operational qualification (OQ) Performance qualification (PQ)
QUALIFICATION
Pharm. (Mrs) Edosa Ogbeide 22

Commissioning • Include equipment startup, adjustments, finetuning, cycle development, testing and documentation flexibility. • Leaves the equipment in a “State of Control”, which ensures the equipment is ready formal qualification, testing, and approval. • Supplements validity and regulatory compliance • checks to verify the system or equipment is built to design specifications • No safety issues • Takes place at the manufacturer’s premise (FAT) or User/Client’s Premise (SAT)

Design qualification (DQ) • Process of completing and documenting design reviews to illustrate that all quality aspects have been fully considered at the design stage. • To ensure that all the requirements for the final systems have been clearly defined at the start. • In other words, has it been designed and selected correctly?

Design qualification (DQ) Before purchasing, a manufacturing equipment • Collection of data about similar equipment available in the market • Assessing your needs • Resources to buy, operate, space and maintenance they would need, etc. • Making the decision

Installation Qualification (IQ) • Process of checking the installation, to ensure that the components meet the approved specification and are installed correctly, and to see how that information is recorded. • To ensure that all aspects (static attributes) of the facility or equipment are installed correctly and comply with the original design.

Installation Qualification (IQ) Considerations include: • Equipment design features (i. e. materials of construction cleanability, etc. ) • Installation conditions (functionality, utilities, wiring, etc. ) • Calibration, preventative maintenance, cleaning schedules; safety features • Supplier documentation, prints, drawings and manuals, software documentation • Environmental conditions (such as clean room requirements, temperature, humidity) • Spare parts list

Installation Qualification (IQ) E. g. , a manufacturing equipment • After purchasing (or critical repair) • Place it on its intended place • Connect with other equipment, electric power, material flow devices. • Collect its documents including Operation Manual, etc. • Its formally “released”: it is ready for working with

Operational Qualification • Process of testing to ensure that the individual and combined systems function to meet agreed performance criteria and to check how the result of testing is recorded. • To ensure that all the dynamic attributes comply with the original design. • Does it work correctly?

Operational Qualification • Assuring that the critical components and systems are capable of operating within established limits and tolerances • “Worst case” (Proven Acceptable Range) demonstration that the equipment will perform as expected while operating at the extremes of the proposed range of operation.

Operational Qualification Considerations include: • Process control limits (e. g. time, temperature, pressure, line speed, setup conditions) • Software parameters; starting material specifications • Process operating procedures; material handling requirements • Process change control; training; short term stability and capability of the process, (latitude studies or control charts) • Risk analysis and potential failure modes, action levels and worst-case conditions (Failure Mode and Effects Analysis, Fault Tree Analysis

Operational Qualification E. g. , a manufacturing equipment • ”Model manufacturing” experiments with model materials, similar to those to be used in the real manufacture • E. g. qualifying an autoclave, we use culture-media • Permit the acceptable fluctuations of parameters, even set the ”worst conditions”

Operational Qualification Worst Conditions • For example, if an equipment is to be operated within the limits of: – Temperature: 20 and 35 o. C – Pressure: 0. 9 and 1. 2 atm • The worst cases are when it operates at – 20 o. C and 0. 9 atm – 35 o. C and 1. 2 atm – 20 o. C 0. 9 and 1. 2 atm – 35 o. C and 0. 9 atm

Performance Qualification • Also called process qualification • Process of testing to ensure that the individual and combined systems function to meet agreed performance criteria on a consistent basis and to check how the result of testing is recorded. • To ensure that the criteria specified can be achieved on a reliable basis over a period of time. • Does it produce the product correctly.

Performance Qualification PQ includes: • Actual product and process parameters and procedures established in OQ • Assurance of process capability as established in OQ • Acceptability of the product • Process repeatability, long term process stability

Performance Qualification • Similar to the Operational Qualification, but the real manufacture is running • Permits accepted fluctuations up to their limits (incl. worse conditions, if occur) • Integrates procedure, personnel, systems, and materials to verify that the pharmaceutical grade utility, environment, equipment, or support system produces the required output • Production is done under conditions that simulate those planned to be used during actual manufacturing

Change Control • • Policy and procedure Risk assessment Authorization Failure to properly document changes to the system means invalidation of the process

Change Control Changes that require revalidation • • Software changes; Controllers Site changes; Operational changes Change of source of material Change in the process Significant equipment change Production area changes Support system changes
Validation Types • Process Validation • Analytical Method Validation • Cleaning Validation • Water Systems Validation • Computerized System Validation

Process Validation • Means of ensuring, and providing documentary evidence that processes (within their specified design parameters) are capable of repeatedly and reliably producing a finished product of the required quality. • PV should be completed prior to commercial manufacturing • Where this is not possible, it may be necessary to validate processes during routine production

Approaches to Validation Prospective Validation • Prospective validation is carried out during the development stage. • Includes division of the production process into separate steps • Analysis of potentially critical points in the manufacturing process e. g. mixing times, or temperature. • Trials are carried out in which these steps and critical points are simulated and the effect on the process is assessed.

Approaches to Validation Prospective Validation q Each step should be evaluated on the basis of experience or theoretical considerations to determine the critical factors/parameters that may affect the quality of the finished product. q May incorporate a challenge element to determine the robustness of the process. q Such a challenge is generally referred to as a "worst case" exercise. Pharm. (Mrs) Edosa Ogbeide q Representatives from Production, QC/QA, Engineering, and in some cases Research and Development (if available) will normally be involved in this process. 42
Approaches to Validation Concurrent Validation • Carried out during normal production. • Requires full understanding of the process based on prospective work. • Involves very close and intensified monitoring of the steps and critical points in at least the first three production-scale batches

Approaches to Validation q Documentation Concurrent Validation requirements are the q In certain same as specified for circumstances it may Prospective Validation not be possible to and the testing to be complete a validation carried out in-process programme before and on the finished routine production product will be as q It is important however, starts. In these cases it specified in approved that the premises and will be known in protocols. equipment to be used advance that the have been qualified q The completed finished product will be previously and that the protocols and reports for sale or supply. decision to carry out should be reviewed Concurrent Validation is and approved before made by appropriately product is released for authorised people. sale or supply. Pharm. (Mrs) Edosa Ogbeide 44

Approaches to Validation Retrospective Validation • The analysis of accumulated results from past production to assess the consistency of a process. • Includes trend analysis on test results and a close examination of all recorded process deviations. • It is important to analyze 10 to 25 batches manufactured over a period of 12 months to provide a statistically significant picture. • It is not the preferred method of validation and should be used in exceptional cases only and never for sterile products
Approaches to Validation Retrospective Validation q There are many processes in routine use in many companies that have not undergone a formally documented validation process. q Validation of these processes is possible, q using historical data to provide the necessary documentary evidence that the process is doing what it is believed to do. q This type of validation exercise is only Data acceptable for well established processes and will be inappropriate where there have been recent The steps involved in this changes in the composition of the type of validation still require the preparation product, operating procedures or of a protocol, the equipment. reporting of the results of the data review, leading to a conclusion and recommendation. Pharm. (Mrs) Edosa Ogbeide 46
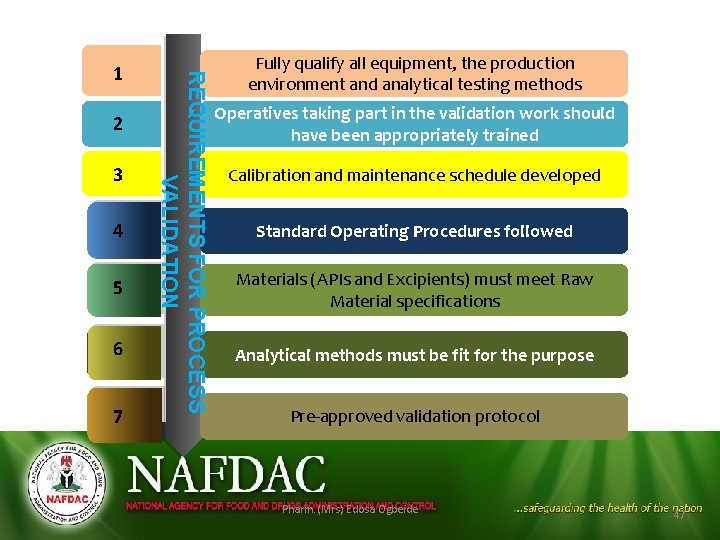
2 3 4 5 6 7 REQUIREMENTS FOR PROCESS VALIDATION 1 Fully qualify all equipment, the production environment and analytical testing methods Operatives taking part in the validation work should have been appropriately trained Calibration and maintenance schedule developed Standard Operating Procedures followed Materials (APIs and Excipients) must meet Raw Material specifications Analytical methods must be fit for the purpose Pre-approved validation protocol Pharm. (Mrs) Edosa Ogbeide 47
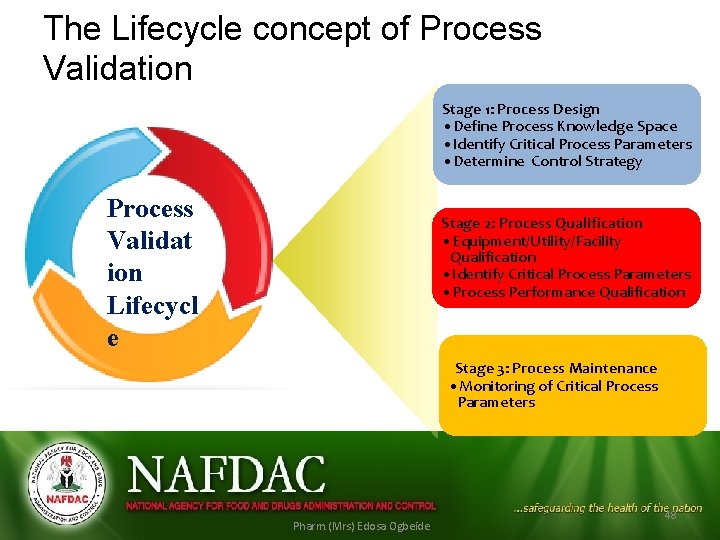
The Lifecycle concept of Process Validation Stage 1: Process Design • Define Process Knowledge Space • Identify Critical Process Parameters • Determine Control Strategy Process Validat ion Lifecycl e Stage 2: Process Qualification • Equipment/Utility/Facility Qualification • Identify Critical Process Parameters • Process Performance Qualification Stage 3: Process Maintenance • Monitoring of Critical Process Parameters Pharm. (Mrs) Edosa Ogbeide 48

The Lifecycle concept of Process Validation • The Lifecycle concept links product and process design, qualification of the commercial manufacturing process and maintenance of the process in a state of control during routine production • A science- and risk-based approach to verify and demonstrate that a process operating within predefined specified parameters consistently produces material that meets all its critical quality attributes.
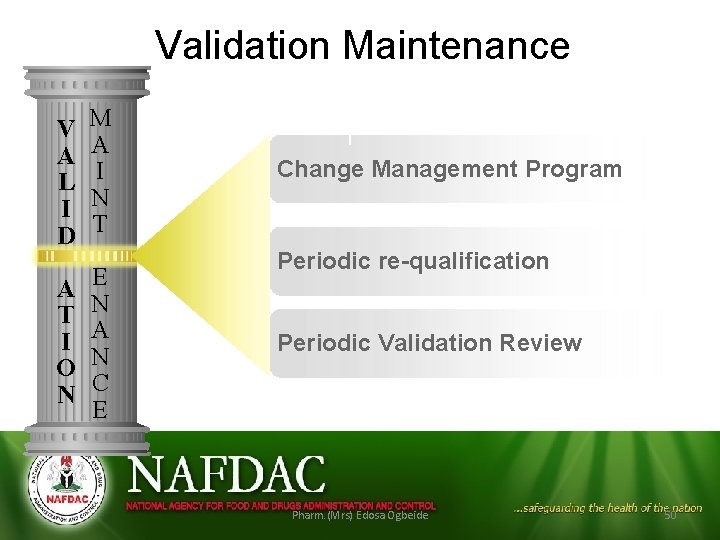
Validation Maintenance V A L I D M A I N T A T I O N E N A N C E 1 Change 2 Management Program 3 re-qualification Periodic Validation Review Pharm. (Mrs) Edosa Ogbeide 50

Validation Maintenance • Change Management is the primary mechanism by which validation is maintained • Effective change control system must be in place to control changes to validated systems • Changes should be formally documented and approved before implementation and records maintained • Commitment of the company to control is essential to ensure a continued validation status of the systems concerned.

Validation Maintenance Revalidation Øchange in operating parameters Øcomponent specifications have changed Ønew accessories or components are added to previously qualified equipment Øprocess changes that potentially impact product effectiveness or quality

Decommissioning • Documented demonstration that at the time of removal from routine operational use, system was operating in compliance with specific requirements and fit for purpose • Should be planned • Requirements depends on system complexity • Outcome should be reported and provide conclusion

Continuous Process Verification • Introduced to cover an alternative approach to process validation based on a continuous monitoring of manufacturing performance • Based on the knowledge from product and process development studies and / or previous manufacturing experience. • Can be introduced at any time in the lifecycle of the product. It can be used for the initial commercial production, to re-validate commercialized products as part of process changes or to support continual improvement

Continuous Process Verification • Involves extensive in-line, on-line or at-line controls and monitoring process performance and product quality on each batch. • Relevant data on quality attributes of incoming materials or components, in-process material and finished products should be collected. • Verification of attributes, parameters and end points, and assessment of CQA and critical process parameter (CPP) trends

Cleaning Validation • Establishing documented evidence that the equipment is consistently cleaned of product, microbial and cleaning agent residues to predetermined acceptable levels • Provides a high degree of assurance that the Cleaning procedure can effectively remove residues of a product and a cleaning agent from the manufacturing equipment, to a level that does not raise patient safety concerns

Cleaning Validation Basic Considerations • Cleaning mechanisms: Mech. action, dissolution, chem. reaction, detergency • Cleaning Procedure: SOPs, tools & materials • Cleaning method: CIP, COP, manual cleaning • Determination of contamination limits: TD, blanket spec. • Worst case selection • Clean Hold times • Sampling: swab, rinse, location, surface area, swab recovery, analytical method

Analytical Method Validation ICH Q 2 (R 1) • Specificity • Linearity • Accuracy • Precision • Robustness • LOD • LOQ

Water Systems Validation Conducted in 3 phases: • Phase 1: 2 -4 weeks - intensive system monitoring, develop operating ranges, implement & refine SOPs • Phase 2: 2 -4 weeks – deploy refined SOPs, demonstrate operation within established ranges, demonstrate production of required quality & quantity • Phase 3: 1 year – verify long-term control, demonstrate consistent production of required quality & quantity • Ongoing system monitoring
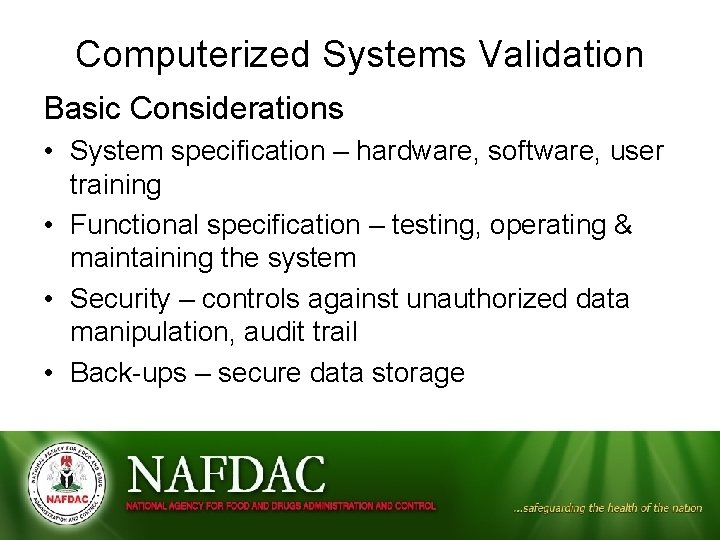
Computerized Systems Validation Basic Considerations • System specification – hardware, software, user training • Functional specification – testing, operating & maintaining the system • Security – controls against unauthorized data manipulation, audit trail • Back-ups – secure data storage

Conclusion § Validation is an essential component of GMP § Helps to achieve goal of is assuring product quality, safety and efficacy § Offers huge business benefits § Lifecycle approach covers entire systems and processes and must be structured, planned and documented § Validation maintenance is essential element of validation lifecycle
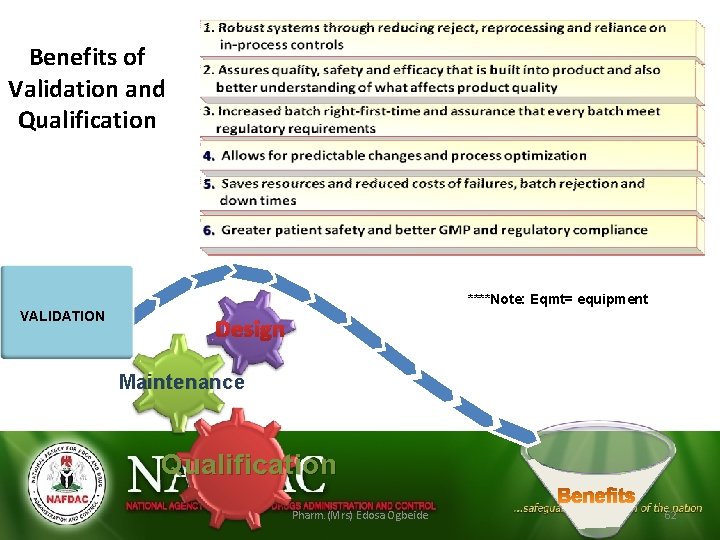
Benefits of Validation and Qualification Eqmt Facilities Utilities Processes VALIDATION ****Note: Eqmt= equipment Design Maintenance Qualification Pharm. (Mrs) Edosa Ogbeide 62

References • NAFDAC c. GMP for Medicinal Products Regulations 2009 • WHO Supplementary Training Modules on GMP. WHO TRS No. 937, 2006. Annex 4 • ICH Tripartite Guidelines: Validation of Analytical Procedures: Text and Methodology Q 2(R 1) • WHO TRS 986 Annex 2; Good manufacturing practices for pharmaceutical products: Main principles • NAFDAC Good manufacturing Practice for Pharmaceutical Products Guidelines 2016 • Chukwumerije O. (2016). Presentation on Pharmaceutical Qualification & Validation • Ogbeide E. (2013). Presentation on Equipment Qualification & Process Validation

THANK YOU

QUESTIONS?
- Slides: 65